Abstract
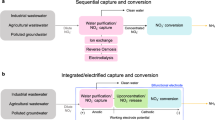
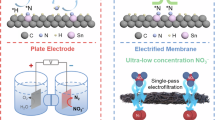
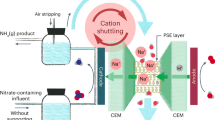
Improving electrochemical reactions by manipulating the properties of catalyst active sites often involves tradeoffs in activity, selectivity, stability and material costs. Here we incorporate a nitrite-adsorbing ionophore as a cooperative nitrite-enriching component into an electrified membrane to achieve high nitrate conversion (94.6%) and ammonia selectivity (91.9%) with a treatment time of only a few seconds (6 s). The ionophore enriched nitrite within the local electrocatalyst environment, facilitating conversion of unreacted nitrite to ammonia to inhibit overall nitrite formation (1.1%) without directly modifying the catalytic active sites. Integrating the ionophore as a selective adsorption component into a copper/carbon nanotube-based electrified membrane led to long-term selective ammonia production from low-concentration nitrate in real surface water and wastewater effluent without using precious metals. The concept of employing cooperative adsorption components to manipulate the local electrocatalyst environment and control reaction selectivity without precious metals or complex synthesis, especially when coupled with the stability and efficiency of scalable electrified membranes, could be extended to advance diverse electrocatalytic applications beyond nitrate.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data are presented in the article and its Supplementary Information. Source data are provided with this paper, including the atomic coordinates of the optimized computational models.
Code availability
The code for generating Fig. 3d,e in the study is publicly available on GitHub at https://github.com/yyan107/Cu_TTM.
References
Wu, Z.-Y. et al. Electrochemical ammonia synthesis via nitrate reduction on Fe single atom catalyst. Nat. Commun. 12, 2870 (2021).
Chen, F.-Y. et al. Efficient conversion of low-concentration nitrate sources into ammonia on a Ru-dispersed Cu nanowire electrocatalyst. Nat. Nanotechnol. 17, 759–767 (2022).
Harmon, N. J., Li, J., Wang, B. T., Gao, Y. & Wang, H. Influence of carbon nanotube support on electrochemical nitrate reduction catalyzed by cobalt phthalocyanine molecules. ACS Catal. 14, 3575–3581 (2024).
Rezayi, M. et al. Titanium(III) cation selective electrode based on synthesized tris(2pyridyl) methylamine ionophore and its application in water samples. Sci. Rep. 4, 4664 (2014).
Cánovas, R., Padrell Sánchez, S., Parrilla, M., Cuartero, M. & Crespo, G. A. Cytotoxicity study of ionophore-based membranes: toward on-body and in vivo ion sensing. ACS Sens. 4, 2524–2535 (2019).
Garcia-Segura, S., Lanzarini-Lopes, M., Hristovski, K. & Westerhoff, P. Electrocatalytic reduction of nitrate: fundamentals to full-scale water treatment applications. Appl. Catal. B 236, 546–568 (2018).
Li, P. et al. Pulsed nitrate-to-ammonia electroreduction facilitated by tandem catalysis of nitrite intermediates. J. Am. Chem. Soc. 145, 6471–6479 (2023).
Néel, B. et al. Nitrite-selective electrode based on cobalt(II) tert-butyl-salophen ionophore. Electroanalysis 26, 473–480 (2014).
Pennino, M. J., Leibowitz, S. G., Compton, J. E., Hill, R. A. & Sabo, R. D. Patterns and predictions of drinking water nitrate violations across the conterminous United States. Sci. Total Environ. 722, 137661 (2020).
C. D’Angelo, S. et al. Environmental and economic potential of decentralised electrocatalytic ammonia synthesis powered by solar energy. Energy Environ. Sci. 16, 3314–3330 (2023).
van Langevelde, P. H., Katsounaros, I. & Koper, M. T. Electrocatalytic nitrate reduction for sustainable ammonia production. Joule 5, 290–294 (2021).
Duca, M. & Koper, M. T. Powering denitrification: the perspectives of electrocatalytic nitrate reduction. Energy Environ. Sci. 5, 9726–9742 (2012).
Gayen, P. et al. Electrocatalytic reduction of nitrate using magnéli phase TiO2 reactive electrochemical membranes doped with Pd-based catalysts. Environ. Sci. Technol. 52, 9370–9379 (2018).
Li, Y., Ma, J., Waite, T. D., Hoffmann, M. R. & Wang, Z. Development of a mechanically flexible 2D-MXene membrane cathode for selective electrochemical reduction of nitrate to N2: mechanisms and implications. Environ. Sci. Technol. 55, 10695–10703 (2021).
Sun, M. et al. Electrified membranes for water treatment applications. ACS EST Eng. 1, 725–752 (2021).
Sarycheva, A. & Gogotsi, Y. Raman spectroscopy analysis of the structure and surface chemistry of Ti3C2Tx MXene. Chem. Mater. 32, 3480–3488 (2020).
Goh, G. L. et al. Potential of printed electrodes for electrochemical impedance spectroscopy (EIS): toward membrane fouling detection. Adv. Electron. Mater. 7, 2100043 (2021).
Ren, G. et al. Membrane electrodes for electrochemical advanced oxidation processes: preparation, self-cleaning mechanisms and prospects. Chem. Eng. J. 451, 138907 (2023).
Sun, J., Hu, C., Wu, B. & Qu, J. Fouling mitigation of a graphene hydrogel membrane electrode by electrical repulsion and in situ self-cleaning in an electro-membrane reactor. Chem. Eng. J. 393, 124817 (2020).
Wu, D. et al. Self-healable metal–organic gel membranes as anodes with high lithium storage. Electrochim. Acta 386, 138334 (2021).
Xu, Y.-T., Xie, M.-Y., Zhong, H. & Cao, Y. In situ clustering of single-atom copper precatalysts in a metal–organic framework for efficient electrocatalytic nitrate-to-ammonia reduction. ACS Catal. 12, 8698–8706 (2022).
Niu, Z. et al. Bifunctional copper-cobalt spinel electrocatalysts for efficient tandem-like nitrate reduction to ammonia. Chem. Eng. J. 450, 138343 (2022).
Zhao, X. et al. Boosting the selectivity and efficiency of nitrate reduction to ammonia with a single-atom Cu electrocatalyst. Chem. Eng. J. 466, 143314 (2023).
Gupta, S., Rivera, D. J., Shaffer, M., Chismar, A. & Muhich, C. Behavior of cupric single atom alloy catalysts for electrochemical nitrate reduction: an ab initio study. ACS EST Eng. 4, 166–175 (2024).
Huang, Y. et al. Real-time in situ monitoring of nitrogen dynamics in wastewater treatment processes using wireless, solid-state, and ion-selective membrane sensors. Environ. Sci. Technol. 53, 3140–3148 (2019).
Gupta, S., Chismar, A., Shaffer, M., Rivera, D. J. & Muhich, C. Surface phenomenon affecting removal efficiency of nitrate from water on dispersed single atoms in Cu metal catalyst: an ab-initio study. in Proc. 2022 AIChE Annual Meeting (AIChE, 2022).
Tran, R. et al. Surface energies of elemental crystals. Sci. Data 3, 1–13 (2016).
Pérez-Gallent, E., Figueiredo, M. C., Katsounaros, I. & Koper, M. T. Electrocatalytic reduction of nitrate on copper single crystals in acidic and alkaline solutions. Electrochim. Acta 227, 77–84 (2017).
Fan, Y. et al. Highly efficient metal-free nitrate reduction enabled by electrified membrane filtration. Nat. Water 2, 684–696 (2024).
Yang, X. et al. MXene-Cu electrified membranes with confined lamellar channels for the flow-through electrochemical reduction of nitrate to ammonia. ACS Sustain. Chem. Eng. 12, 3378–3389 (2024).
Ren, Y. et al. Fluidic MXene electrode functionalized with iron single atoms for selective electrocatalytic nitrate transformation to ammonia. Environ. Sci. Technol. 57, 10458–10466 (2023).
Chen, M. et al. Bi2O3 nanosheets arrays in-situ decorated on carbon cloth for efficient electrochemical reduction of nitrate. Chemosphere 278, 130386 (2021).
Xu, Y. et al. Cooperativity of Cu and Pd active sites in CuPd aerogels enhances nitrate electroreduction to ammonia. Chem. Commun. 57, 7525–7528 (2021).
Cerrón-Calle, G. A., Fajardo, A. S., Sánchez-Sánchez, C. M. & Garcia-Segura, S. Highly reactive Cu–Pt bimetallic 3D-electrocatalyst for selective nitrate reduction to ammonia. Appl. Catal. B 302, 120844 (2022).
Wang, K. et al. Intentional corrosion-induced reconstruction of defective NiFe layered double hydroxide boosts electrocatalytic nitrate reduction to ammonia. Nat. Water 1, 1068–1078 (2023).
Zhang, Y., Chen, X., Wang, W., Yin, L. & Crittenden, J. C. Electrocatalytic nitrate reduction to ammonia on defective Au1Cu(111) single-atom alloys. Appl. Catal. B Environ. 310, 121346 (2022).
Vaziri Rad, M. A., Kasaeian, A., Niu, X., Zhang, K. & Mahian, O. Excess electricity problem in off-grid hybrid renewable energy systems: a comprehensive review from challenges to prevalent solutions. Renew. Energy 212, 538–560 (2023).
Wang, X. et al. Free-standing membrane incorporating single-atom catalysts for ultrafast electroreduction of low-concentration nitrate. Proc. Natl Acad. Sci. USA 120, e2217703120 (2023).
Sauerbrey, G. Verwendung von Schwingquarzen zur Wägung dünner Schichten und zur Mikrowägung. Z. Für Phys. 155, 206–222 (1959).
Shao, Y., Ying, Y. & Ping, J. Recent advances in solid-contact ion-selective electrodes: functional materials, transduction mechanisms, and development trends. Chem. Soc. Rev. 49, 4405–4465 (2020).
Le, T. X. H., Haflich, H., Shah, A. D. & Chaplin, B. P. Energy-efficient electrochemical oxidation of perfluoroalkyl substances using a Ti4O7 reactive electrochemical membrane anode. Environ. Sci. Technol. Lett. 6, 504–510 (2019).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H–Pu. J. Chem. Phys. 132, 154104 (2010).
Mathew, K., Sundararaman, R., Letchworth-Weaver, K., Arias, T. A. & Hennig, R. G. Implicit solvation model for density-functional study of nanocrystal surfaces and reaction pathways. J. Chem. Phys. 140, 084106 (2014).
Mathew, K., Kolluru, V. S., Mula, S., Steinmann, S. N. & Hennig, R. G. Implicit self-consistent electrolyte model in plane-wave density-functional theory. J. Chem. Phys. 151, 234101 (2019).
Nørskov, J. K. et al. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J. Phys. Chem. B 108, 17886–17892 (2004).
Lide, D. R. & Kehiaian, H. V. CRC Handbook of Thermophysical and Thermochemical Data (CRC Press, 2020).
Yan, Y., Masood, Z. & Wang, B. Lanthanum-doped graphene for electrocatalytic reduction of nitrogen monoxide. J. Phys. Chem. C 127, 12967–12975 (2023).
Chan, K. & Nørskov, J. K. Electrochemical barriers made simple. J. Phys. Chem. Lett. 6, 2663–2668 (2015).
Chan, K. & Nørskov, J. K. Potential dependence of electrochemical barriers from ab initio calculations. J. Phys. Chem. Lett. 7, 1686–1690 (2016).
Zhao, Q., Martirez, J. M. P. & Carter, E. A. Revisiting understanding of electrochemical CO2 reduction on Cu(111): competing proton-coupled electron transfer reaction mechanisms revealed by embedded correlated wavefunction theory. J. Am. Chem. Soc. 143, 6152–6164 (2021).
Gordon, M. S. & Schmidt, M. W. in Theory and Applications of Computational Chemistry (eds Dykstra, C. E. et al.) Ch. 14 (Elsevier, 2005).
Bode, B. M. & Gordon, M. S. MacMolPlt: a graphical user interface for GAMESS. J. Mol. Graph. Model. 16, 133–138 (1998).
Stephens, P. J., Devlin, F. J., Chabalowski, C. F. & Frisch, M. J. Ab initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. J. Phys. Chem. 98, 11623–11627 (1994).
Lee, C., Yang, W. & Parr, R. G. Development of the Colle–Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 37, 785–789 (1988).
Cossi, M., Rega, N., Scalmani, G. & Barone, V. Energies, structures, and electronic properties of molecules in solution with the C‐PCM solvation model. J. Comput. Chem. 24, 669–681 (2003).
Barone, V. & Cossi, M. Quantum calculation of molecular energies and energy gradients in solution by a conductor solvent model. J. Phys. Chem. A 102, 1995–2001 (1998).
Nwokonkwo, O., Pelletier, V., Broud, M. & Muhich, C. Functionalized ferrocene enables selective electrosorption of arsenic oxyanions over phosphate—a DFT examination of the effects of substitutional moieties, pH, and oxidation State. J. Phys. Chem. A 127, 7727–7738 (2023).
Gani, T. Z. H., Ioannidis, E. I. & Kulik, H. J. Computational discovery of hydrogen bond design rules for electrochemical ion separation. Chem. Mater. 28, 6207–6218 (2016).
Acknowledgements
This research was supported by the NSF Nanosystems Engineering Research Center for Nanotechnology-Enabled Water Treatment (grant no. EEC-1449500 to L.R.W.). The computations were conducted through the Arizona State University research computing environment. We thank Y. Duan (Yale University) for discussions on electrofiltration mechanisms.
Author information
Authors and Affiliations
Contributions
Y.F., X.W. and L.R.W. conceived of the idea and designed the experiments. L.R.W. supervised the project. Y.F., E.C. and J.S. fabricated the membranes. Y.F., E.C., J.S., J.-Y.K., M.S.-M. and W.P. performed the membrane tests and analyzed the results. Y.F., Y.Y., O.N., D.J.R. and C.M. carried out the DFT calculations and analysis. Y.F., Y.Y. and L.R.W. wrote the paper. All authors discussed the results and revised the paper.
Corresponding author
Ethics declarations
Competing interests
Y.F. and L.R.W. are listed as coinventors on International Patent Application No. PCT/US25/27226 filed on 1 May 2025, submitted by Yale University, which covers the coupling electrofiltration with a cooperative nitrite-enriching component for nitrate reduction as described in this paper. The other authors declare no competing interests.
Peer review
Peer review information
Nature Chemical Engineering thanks Bryan Goldsmith and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–33, Tables 1–9 and Notes 1–4.
Supplementary Data 1
Atomic coordinates of the optimized computational models for CuNP in Fig. 3c.
Supplementary Data 2
Atomic coordinates of the optimized computational models for TTM in Fig. 3c.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fan, Y., Yan, Y., Nwokonkwo, O. et al. Tuning nitrate reduction reaction selectivity via selective adsorption in electrified membranes. Nat Chem Eng 2, 379–390 (2025). https://doi.org/10.1038/s44286-025-00237-3
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s44286-025-00237-3
This article is cited by
-
Managing electrolyte flow boosts the efficiency of continuous oxime electrosynthesis to over 95%
Nature Communications (2026)
-
Robust catalyst assessment for the electrocatalytic nitrate reduction reaction
Communications Chemistry (2025)