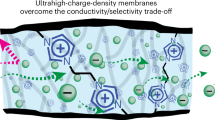
Abstract
Ion-selective membranes are widely used in water treatment and batteries. However, it is challenging to obtain membranes that are both selective and permeable. Here, we report an interfacial polymer cross-linking strategy to produce ultrathin but robust polymeric membranes that are simultaneously permeable and selective. Cross-linking the polymer at the interface of two immiscible solvents followed by nonsolvent exchange produces a 3-µm-thick ultrathin membrane that contains a nanoscale separation layer with a quasi-ordered reticular cross-linking structure. Besides conferring strength, the cross-linked structures have angstrom-scale channels and ion-selective sites that can precisely separate ions of similar sizes and charges. We show that these membranes enable increased working current density and power density of various aqueous flow batteries. This strategy resolves a long-standing challenge in polymeric membranes.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All the data supporting the results in this study are available within the Article and its Supplementary Information. Source data are provided with this paper.
References
Joseph, N., Ahmadiannamini, P., Hoogenboom, R. & Vankelecom, J. Layer-by-layer preparation of polyelectrolyte multilayer membranes for separation. Polym. Chem. 5, 1817–1831 (2014).
Chen, G. et al. Zeolites and metal–organic frameworks for gas separation: the possibility of translating adsorbents into membranes. Chem. Soc. Rev. 52, 4586–4602 (2023).
Zhang, H., He, Q., Luo, J., Wan, Y. & Darling, S. B. Sharpening nanofiltration: strategies for enhanced membrane selectivity. ACS Appl. Mater. Interfaces 12, 39948–39966 (2020).
Geise, G. M. Why polyamide reverse-osmosis membranes work so well. Science 371, 31–32 (2021).
Culp, T. E. et al. Nanoscale control of internal inhomogeneity enhances water transport in desalination membranes. Science 371, 72–75 (2021).
Uliana, A. A. et al. Ion-capture electrodialysis using multifunctional adsorptive membranes. Science 372, 296–299 (2021).
Kidambi, P. R., Chaturvedi, P. & Moehring, N. K. Subatomic species transport through atomically thin membranes: present and future applications. Science 374, 708 (2021).
Chen, G. et al. Solid-solvent processing of ultrathin, highly loaded mixed-matrix membrane for gas separation. Science 381, 1350–1356 (2023).
Datta, S. et al. Rational design of mixed-matrix metal-organic framework membranes for molecular separations. Science 376, 1080–1087 (2022).
Qian, Q. et al. MOF-based membranes for gas separations. Chem. Rev. 120, 8161–8266 (2020).
Tan, X. et al. Truly combining the advantages of polymeric and zeolite membranes for gas separations. Science 378, 1189–1194 (2022).
Robeson, L. M. Correlation of separation factor versus permeability for polymeric membranes. J. Membr. Sci. 62, 165–185 (1991).
Wang, A. et al. Selective ion transport through hydrated micropores in polymer membranes. Nature 635, 353–358 (2024).
Huang, T. et al. Molecularly-porous ultrathin membranes for highly selective organic solvent nanofiltration. Nat. Commun. 11, 5882 (2020).
Karan, S., Jiang, Z. & Livingston, A. G. Sub-10 nm polyamide nanofilms with ultrafast solvent transport for molecular separation. Science 348, 1347–1351 (2015).
Zhang, S. et al. Ultrathin membranes for separations: a new era driven by advanced nanotechnology. Adv. Mater. 34, 2108457 (2022).
Jiang, Z. et al. Aligned macrocycle pores in ultrathin films for accurate molecular sieving. Nature 609, 58–64 (2022).
Sandru, M. et al. An integrated materials approach to ultrapermeable and ultraselective CO2 polymer membranes. Science 376, 90–94 (2022).
Sengupta, B. et al. Carbon-doped metal oxide interfacial nanofilms for ultrafast and precise separation of molecules. Science 381, 1098–1104 (2023).
Gao, S., Wang, D., Fang, W. & Jin, J. Ultrathin membranes: a new opportunity for ultrafast and efficient separation. Adv. Mater. Technol. 5, 1901069 (2020).
Sarkar, P., Wu, C., Yang, Z. & Tang, C. Y. Empowering ultrathin polyamide membranes at the water–energy nexus: strategies, limitations, and future perspectives. Chem. Soc. Rev. 53, 4374–4399 (2024).
Lu, X. & Elimelech, M. Fabrication of desalination membranes by interfacial polymerization: history, current efforts, and future directions. Chem. Soc. Rev. 50, 6290–6307 (2021).
Choi, J. et al. Thin film composite membranes as a new category of alkaline water electrolysis membranes. Small 19, 2300825 (2023).
Huang, L. & McCutcheon, J. R. Impact of support layer pore size on performance of thin film composite membranes for forward osmosis. J. Membr. Sci. 483, 25–33 (2015).
Xia, Y. et al. Polymeric membranes with aligned zeolite nanosheets for sustainable energy storage. Nat. Sustain. 5, 1080–1091 (2022).
Wu, J., Dai, Q., Zhang, H. & Li, X. A defect-free MOF composite membrane prepared via in-situ binder-controlled restrained second-growth method for energy storage device. Energy Stor. Mater. 35, 687–694 (2021).
Zhu, J. et al. A molecular-sieve electrolyte membrane enables separator-free zinc batteries with ultralong cycle life. Adv. Mater. 34, 2207209 (2022).
Tang, L., Li, T., Lu, W. & Li, X. Lamella-like electrode with high Br2-entrapping capability and activity enabled by adsorption and spatial confinement effects for bromine-based flow battery. Sci. Bull. 67, 1362–1371 (2022).
Hu, J., Zhang, H., Xu, W., Yuan, Z. & Li, X. Mechanism and transfer behavior of ions in Nafion membranes under alkaline media. J. Membr. Sci. 566, 8–14 (2018).
Hu, J. et al. Layered double hydroxide membrane with high hydroxide conductivity and ion selectivity for energy storage device. Nat. Commun. 12, 3409 (2021).
Zhao, Q. H. et al. A new digital positron annihilation lifetime spectrometer for a single piece of micron-thickness film. Nuclear Inst. Methods Phys. Res. A. 1038, 166921 (2022).
Zuo, P. et al. Near-frictionless ion transport within triazine framework membranes. Nature 617, 299–305 (2023).
Zhang, Q. et al. Covalent organic framework–based porous ionomers for high-performance fuel cells. Science 378, 181–186 (2022).
Dai, Q. et al. High-performance PBI membranes for flow batteries: from the transport mechanism to the pilot plant. Energy Environ. Sci. 15, 1594–1600 (2022).
Zhang, M., Li, T., Liu, X., Zhang, C. & Li, X. Molecular revealing the high-stable polycyclic azine derivatives for long-lifetime aqueous organic flow batteries. Adv. Funct. Mater. 34, 2312608 (2024).
Liu, Y. et al. Sustainable polyester thin films for membrane desalination developed through interfacial catalytic polymerization. Nat. Water 3, 430–438 (2025).
Chen, Y. W. et al. A non-beam-based Doppler broadening of positron annihilation radiation (DBAR) spectrometer for a single piece of micron-thickness film. Nucl. Instrum. Methods Phys. Res. A 1063, 169286 (2024).
Kansy, J. Microcomputer program for analysis of positron annihilation lifetime spectra. Nucl. Instrum. Methods Phys. Res. 374, 235–244 (1996).
Shukla, A., Peter, M. & Hoffmann, L. Analysis of positron lifetime spectra using quantified maximum entropy and a general linear filter. Nucl. Instrum. Methods Phys. Res. A 335, 310–317 (1993).
Case, D. et al. AMBER 2018 (Univ. California, 2018).
Martínez, L., Andrade, R., Birgin, E. G. & Martínez, J. M. PACKMOL: a package for building initial configurations for molecular dynamics simulations. J. Comput. Chem. 30, 2157–2164 (2009).
Wang, J., Wolf, R. M., Caldwell, J. W., Kollman, P. A. & Case, D. A. Development and testing of a general AMBER force field. J. Comput. Chem. 25, 1157–1174 (2004).
Frisch, M. et al. Gaussian 16 Rev. B.01 (Wallingford, 2016).
Berendsen, H. J. C., Vanderspoel, D. & Vandrunen, R. Gromacs—a message-passing parallel molecular-dynamics implementation. Comput. Phys. Commun. 91, 43–56 (1995).
Darden, T., York, D. & Pedersen, L. Particle mesh Ewald—an N.log(N) method for Ewald sums in large systems. J. Chem. Phys. 98, 10089–10092 (1993).
Roe, D. R. & Cheatham, T. E. PTRAJ and CPPTRAJ: software for processing and analysis of molecular dynamics trajectory data. J. Chem. Theory Comput. 9, 3084–3095 (2013).
Humphrey, W., Dalke, A. & Schulten, K. VMD: visual molecular dynamics. J. Mol. Graph. Model. 14, 33–38 (1996).
Hunter, J. D. Matplotlib: a 2D graphics environment. Comput. Sci. Eng. 9, 90–95 (2007).
Acknowledgements
This work was supported by National Natural Science Foundation of China (grant nos. 22525081 (X. Li), 21925804 (X. Li), 22379141 (W.L.) and 12275270 (H.Z.)), National Key R&D Program of China (grant no. 2022YFB2404901) (W.L.), CAS Strategic Leading Science & Technology Program (A) (grant no. XDA0400201) (X. Li) and Youth Innovation Promotion Association CAS (grant no. 2022184) (W.L.). We thank A. L. Chun of Science Storylab for valuable discussions and for critically reading and editing the manuscript.
Author information
Authors and Affiliations
Contributions
X. Liu, M.S. and W.L. conceptualized and performed the experiments and analyzed the data. C.L. carried out the simulations. N.T. performed the experiments. Y.C. performed the experiments and analyzed the data. C.D. performed the experiments. H.Z. analyzed the data. W.L. and X. Li conceptualized the experiments, conceived and supervised the entire study. X. Liu, M.S. and W.L. were involved in the development of the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemical Engineering thanks Michael Aziz and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Methods section, Figs. 1–73, Tables 1–7 and Refs. 1–55.
Source data
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, X., Shi, M., Liao, C. et al. Ultrathin membranes prepared through interfacial polymer cross-linking for selective and fast ion transport. Nat Chem Eng 2, 369–378 (2025). https://doi.org/10.1038/s44286-025-00238-2
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s44286-025-00238-2