Abstract
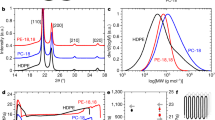
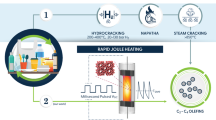
Plastic waste is a growing problem, accumulating in landfills and the environment. Pyrolysis is a promising and industrially relevant approach for transforming plastic waste into value-added chemicals. However, the selectivity and yield of traditional plastic pyrolysis are poor, with products featuring broad molar mass distributions. Here we report a highly selective, energy-efficient and catalyst-free pyrolysis method that can upcycle plastic into value-added chemicals via pore-modulated pyrolysis. Using a Joule-heated carbon column, we demonstrate the pivotal role of the reactor’s graded porous structure in decreasing the polydispersity of the reaction intermediates, enabling high product selectivity and yield. The decreasing pore size of the reactor modulates the mass transport in an apparent gating effect—preventing high-molar-mass species from exiting the reactor before sufficient pyrolysis has occurred. Using polyethylene as a model reactant, we demonstrate a high yield of 65.9 ± 5.2% and up to 80.8% selectivity toward value-added aviation fuel precursor (C8–C18 hydrocarbons) without the use of any catalysts.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Data supporting the findings of this study are available from the corresponding author on request. Source data are provided with this paper.
Change history
14 August 2025
A Correction to this paper has been published: https://doi.org/10.1038/s44286-025-00270-2
References
Geyer, R., Jambeck, J. R. & Law, K. L. Production, use, and fate of all plastics ever made. Sci. Adv. 3, e1700782 (2017).
Vispute, T. P., Zhang, H., Sanna, A., Xiao, R. & Huber, G. W. Renewable chemical commodity feedstocks from integrated catalytic processing of pyrolysis oils. Science 330, 1222–1227 (2010).
Lee, K., Jing, Y., Wang, Y. & Yan, N. A unified view on catalytic conversion of biomass and waste plastics. Nat. Rev. Chem. 6, 635–652 (2022).
Madanikashani, S., Vandewalle, L. A., De Meester, S., De Wilde, J. & Van Geem, K. M. Multi-scale modeling of plastic waste gasification: opportunities and challenges. Materials 15, 4215 (2022).
Lopez, G. et al. Recent advances in the gasification of waste plastics. A critical overview. Renew. Sustain. Energy Rev. 82, 576–596 (2018).
Qiu, Z. et al. A reusable, impurity-tolerant and noble metal–free catalyst for hydrocracking of waste polyolefins. Sci. Adv. 9, eadg5332 (2023).
Zhang, F. et al. Polyethylene upcycling to long-chain alkylaromatics by tandem hydrogenolysis/aromatization. Science 370, 437–441 (2020).
Jia, C. H. et al. Deconstruction of high-density polyethylene into liquid hydrocarbon fuels and lubricants by hydrogenolysis over Ru catalyst. Chem Catal. 1, 437–455 (2021).
Muhammad, C., Onwudili, J. A. & Williams, P. T. Catalytic pyrolysis of waste plastic from electrical and electronic equipment. J. Anal. Appl. Pyrol. 113, 332–339 (2015).
Lopez, G., Artetxe, M., Amutio, M., Bilbao, J. & Olazar, M. Thermochemical routes for the valorization of waste polyolefinic plastics to produce fuels and chemicals. A review. Renew. Sustain. Energy Rev. 73, 346–368 (2017).
Sidhu, N. et al. On the intrinsic reaction kinetics of polypropylene pyrolysis. Matter 6, 3413–3433 (2023).
Mastalski, I. et al. Intrinsic millisecond kinetics of polyethylene pyrolysis via pulse-heated analysis of solid reactions. Chem. Mater. 35, 3628–3639 (2023).
Honus, S., Kumagai, S., Fedorko, G., Molnár, V. & Yoshioka, T. Pyrolysis gases produced from individual and mixed PE, PP, PS, PVC, and PET—part I: production and physical properties. Fuel 221, 346–360 (2018).
Li, H. et al. Hydroformylation of pyrolysis oils to aldehydes and alcohols from polyolefin waste. Science 381, 660–666 (2023).
Ragaert, K., Delva, L. & Van Geem, K. Mechanical and chemical recycling of solid plastic waste. Waste Manag. 69, 24–58 (2017).
Stegmann, P., Daioglou, V., Londo, M., van Vuuren, D. P. & Junginger, M. Plastic futures and their CO2 emissions. Nature 612, 272–276 (2022).
Mallapragada, D. S. et al. Decarbonization of the chemical industry through electrification: barriers and opportunities. Joule 7, 23–41 (2023).
Dong, Q. et al. Programmable heating and quenching for efficient thermochemical synthesis. Nature 605, 470–476 (2022).
Tennakoon, A. et al. Catalytic upcycling of high-density polyethylene via a processive mechanism. Nat. Catal. 3, 893–901 (2020).
Vollmer, I., Jenks, M. J. F., Gonzalez, R. M., Meirer, F. & Weckhuysen, B. M. Plastic waste conversion over a refinery waste catalyst. Angew. Chem. Int. Ed. 60, 16101–16108 (2021).
Martin, A. J., Mondelli, C., Jaydev, S. D. & Perez-Ramirez, J. Catalytic processing of plastic waste on the rise. Chem 7, 1487–1533 (2021).
Ellis, L. D. et al. Chemical and biological catalysis for plastics recycling and upcycling. Nat. Catal. 4, 539–556 (2021).
Van Geem, K. M., Galvita, V. V. & Marin, G. B. Making chemicals with electricity. Science 364, 734–735 (2019).
Chen, S. et al. Ultrasmall amorphous zirconia nanoparticles catalyse polyolefin hydrogenolysis. Nat.Catal. 6, 161–173 (2023).
Jia, X., Qin, C., Friedberger, T., Guan, Z. & Huang, Z. Efficient and selective degradation of polyethylenes into liquid fuels and waxes under mild conditions. Sci. Adv. 2, e1501591 (2016).
Dogu, O. et al. The chemistry of chemical recycling of solid plastic waste via pyrolysis and gasification: state-of-the-art, challenges, and future directions. Prog. Energy Combust. Sci. 84, 100901 (2021).
Zhang, W. et al. Low-temperature upcycling of polyolefins into liquid alkanes via tandem cracking-alkylation. Science 379, 807–811 (2023).
Du, J. et al. Efficient solvent- and hydrogen-free upcycling of high-density polyethylene into separable cyclic hydrocarbons. Nat. Nanotechnol. 18, 772–779 (2023).
Butt, J. B. & Petersen, E. E. in Activation, Deactivation, And Poisoning Of Catalysts (eds Butt, J. B. & Petersen, E. E.) 237–244 (Elsevier, 1988).
Hemberger, P., Custodis, V. B. F., Bodi, A., Gerber, T. & van Bokhoven, J. A. Understanding the mechanism of catalytic fast pyrolysis by unveiling reactive intermediates in heterogeneous catalysis. Nat. Commun. 8, 15946 (2017).
Venderbosch, R. & Prins, W. Fast pyrolysis technology development. Biofuels, Bioprod. Bioref. 4, 178–208 (2010).
Zolghadr, A. et al. On the method of pulse-heated analysis of solid reactions (PHASR) for polyolefin pyrolysis. ChemSusChem 14, 4214–4227 (2021).
Dong, Q. et al. Depolymerization of plastics by means of electrified spatiotemporal heating. Nature 616, 488–494 (2023).
Xu, Z. et al. Chemical upcycling of polyethylene, polypropylene, and mixtures to high-value surfactants. Science 381, 666–671 (2023).
Xu, Z. et al. Cascade degradation and upcycling of polystyrene waste to high-value chemicals. Proc. Natl Acad. Sci. USA 119, e2203346119 (2022).
Cussler, E. L. in Diffusion: Mass Transfer In Fluid Systems 56–95 (Cambridge Univ. Press, 2009).
Yoshida, J., Nagaki, A., Iwasaki, T. & Suga, S. Enhancement of chemical selectivity by microreactors. Chem. Eng. Technol. 28, 259–266 (2005).
Feng, J. et al. Micelles cascade assembly to tandem porous catalyst for waste plastics upcycling. Angew. Chem. Int. Ed. 63, e202405252 (2024).
Elnashaie, S. S. E. H. in Modelling, Simulation And Optimization Of Industrial Fixed Bed Catalytic Reactors 1st edn, 137–145 (CRC Press, 1994).
Tian, Y. & Wang, W. in Diameter-Transformed Fluidized Bed: Fundamentals And Practice (eds Xu, Y. et al.) 49–69 (Springer International Publishing, 2020).
Cen, Z. et al. Upcycling of polyethylene to gasoline through a self-supplied hydrogen strategy in a layered self-pillared zeolite. Nat. Chem. 16, 871–880 (2024).
Wang, S. et al. Ultra-narrow alkane product distribution in polyethylene waste hydrocracking by zeolite micropore diffusion optimization. Angew. Chem. Int. Ed. 2024, e202409288 (2024).
Selvam, E., Yu, K., Ngu, J., Najmi, S. & Vlachos, D. G. Recycling polyolefin plastic waste at short contact times via rapid Joule heating. Nat. Commun. 15, 5662 (2024).
Schobert, H. H. in The Chemistry Of Hydrocarbon Fuels 85–112 (Butterworth-Heinemann, 2013).
Jones, S. et al. Process design and economics for the conversion of lignocellulosic biomass to hydrocarbon fuels: fast pyrolysis and hydrotreating bio-oil pathway. https://doi.org/10.2172/1126275 (2013).
Yadav, G. et al. Techno-economic analysis and life cycle assessment for catalytic fast pyrolysis of mixed plastic waste. Energy Environ. Sci. 16, 3638–3653 (2023).
Schäppi, R. et al. Drop-in fuels from sunlight and air. Nature 601, 63–68 (2022).
Zhang, C. et al. Carbon additive manufacturing with a near-replica “green-to-brown” transformation. Adv. Mater. 35, 2208230 (2023).
Xie, H. et al. A stable atmospheric-pressure plasma for extreme-temperature synthesis. Nature 623, 964–971 (2023).
Mumtaz, F., Zhang, B., O’Malley, R. J. & Huang, J. Large-scale cascading of first-order FBG array in a highly multimode coreless fiber using femtosecond laser for distributed thermal sensing. Opt. Express 31, 29639–29653 (2023).
Chen, C., Garedew, M. & Sheehan, S. W. Single-step production of alcohols and paraffins from CO2 and H2 at metric ton scale. ACS Energy Lett. 7, 988–992 (2022).
Gracida-Alvarez, U. R., Winjobi, O., Sacramento-Rivero, J. C. & Shonnard, D. R. System analyses of high-value chemicals and fuels from a waste high-density polyethylene refinery. Part 1: conceptual design and techno-economic assessment. ACS Sustain. Chem. Eng. 7, 18254–18266 (2019).
Ma, D. Transforming end-of-life plastics for a better world. Nat. Sustain. 6, 1142–1143 (2023).
Kwon, D. Three ways to solve the plastics pollution crisis. Nature 616, 234–237 (2023).
Acknowledgements
We extend our gratitude to H. Liu, X. Zhao (Michigan State University), B. Mei (Princeton University), S. Li (University of Maryland) and X. Wang (University of California, Irvine) for insightful discussions regarding this study. J.Y., L.H. and Y.J. acknowledge financial support from the US Department of Energy (DOE), Office of Science, Basic Energy Sciences, under award no. DE-SC0023357. L.H. and Y.J. acknowledge financial support from the US DOE, Office of Science Energy Earthshot Initiative as part of the Non-equilibrium Energy Transfer for Efficient Reactions (NEETER) at Oak Ridge National Laboratory, under contract no. DE-AC05-00OR22725 and Plasma-Enhanced H2 Production (PEHPr) Energy Earthshot Research Center (EERC) at Princeton Plasma Physics Laboratory under contract no. DE-AC0209CH11466 and US DOE, Basic Energy Sciences, under award no. DE-SC0021135. K.F. and C.Z. acknowledge funding support from the University of Delaware, technical support from CarbonForm Inc., and grant support from the US DOE, Office of Fossil Energy and Carbon Management, under award no. DE-FE0032147. W.Z. and S.H. gratefully acknowledge financial support provided by the National Science Foundation via award no. CBET-2055416 for the part of the work regarding transient process modeling and simulation. S.H. acknowledges the Yale Planetary Solutions seed grant program for financial support. J.C., Z.P. and X.P. acknowledge funding from United States Department of Agriculture (USDA)–National Institute of Food and Agriculture (NIFA) projects (2022-67021-37602 and 2023-68016-38933) and USDA–NIFA Hatch project (WIS05061). F.V.L., C.A.N. and B.D. acknowledge financial support provided by the National Science Foundation RII Track-2 FEC (OIA-2119688). J.H. is grateful for support from the Roy A. Wilkens Professorship Endowment. This work was authored in part by the National Renewable Energy Laboratory for the US DOE under contract no. DE-AC36-08GO28308. Funding was provided to J.M. and G.T.B. by the US DOE, Office of Energy Efficiency and Renewable Energy, Advanced Materials and Manufacturing Technologies Office, and Bioenergy Technologies Office. This work was performed as part of the Bio-Optimized Technologies to keep Thermoplastics out of Landfills and the Environment (BOTTLE) Consortium. The views expressed in the article do not necessarily represent the views of the DOE or the US government. The US government retains and the publisher, by accepting the article for publication, acknowledges that the US government retains a non-exclusive, paid-up, irrevocable, worldwide license to publish or reproduce the published form of this work, or allow others to do so, for US government purposes.
Author information
Authors and Affiliations
Contributions
L.H., J.Y. and Q.D. conceived the idea and designed the research work. J.Y., Q.D., S.L., Y.D. and T.L. performed the plastic pyrolysis experiments. C.Z. and K.F. were responsible for the design, optimization and fabrication of the 3D-printed pyrolysis reactor. The optimization and design of the pyrolysis reaction apparatus were carried out by L.H., J.Y. and Q.D. Simulation and calculation of the pyrolysis process were carried out by S.H. and W.Z. Analytical work on the liquid product composition and yield was conducted by Z.P., J.C. and X.P. Analysis of the gaseous product components was performed by Z.L. and D.L. Precise temperature measurements during the pyrolysis process were conducted by J.Y., N.L., Y.J., B.Z., F.M. and J.H. The simulation of temperature distribution and heat and mass transfer during the pyrolysis process were executed by X.Z. and H.L. Combustion and fuel properties of the pyrolysis products were tested by N.L., Z.W. and Y.J. The study of molar mass and distribution of intermediates in the pyrolysis process was undertaken by J.M. and G.T.B. Economic and technical analyses of the pyrolysis process were conducted by C.A.N., B.D. and F.V.L. J.Y., L.H., Q.D. and A.H.B. wrote the paper. All authors discussed the results and contributed to the final paper.
Corresponding authors
Ethics declarations
Competing interests
L.H., J.Y., Q.D. and S.L. report a patent application titled ‘System, device, and method for converting plastics and natural macromolecules to useful chemicals’ filed on June 14, 2023, US application no. PCT/US2023/63508181. K.F. and C.Z. report a patent application titled ‘Process and system for additive manufacturing of carbon scaffolds’ filed on April 20, 2023, International application no. PCT/US2023/019227. The other authors declare no competing interests.
Peer review
Peer review information
Nature Chemical Engineering thanks James Tour, Ning Yan and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 3D-printing process for fabricating the graded-pore pyrolysis reactor.
a, The 3D-printing process begins with preparing a solution of polylactic acid (PLA) and dichloromethane (DCM) by dissolving 42 grams of PLA in 200 milliliters of DCM at 70 °C. Once dissolved, 18 grams of carboxylic acid (–COOH) functionalized multi-walled carbon nanotubes (MWCNTs) are dispersed into the mixture, followed by further heating to remove DCM, yielding a 30/70 wt.% MWCNT/PLA composite. This composite is then extruded into a filament with a 1.75 mm diameter for 3D printing. The printing parameters are outlined in Supplementary Table 2. Post-printing, carbonization is performed at 600 °C in a nitrogen atmosphere to fully convert the MWCNT/PLA composites into carbon structures. b, Digital photos of each step of the 3D-printing process of the pyrolysis reactor.
Extended Data Fig. 2 Surface and SEM internal structure characterization comparing a newly fabricated pyrolysis reactor and one tested for 10 hours under the same heating conditions as the pyrolysis experiments.
We conducted a 10-hour durability test on the fabricated pyrolysis reactor under the same conditions as the pyrolysis experiments (25 V applied to achieve a peak temperature of 630 °C in Zone 1, with argon as the carrier gas at a flow rate of 40 sccm). (a) Visual inspection of the reactor surfaces showed that they remained smooth, with no signs of cracks or wear after the extended testing period. Further SEM analysis provided detailed views of the internal structures before (b) and after (c) 10 hours of operation, revealing no noticeable changes or degradation despite prolonged exposure to pyrolytic conditions.
Extended Data Fig. 3 Experimental setup for the graded-pore pyrolysis reactor.
a, The 3D-printed graded-pore reactor was integrated with electrical circuitry to provide Joule heating for pyrolysis. Additionally, the reactor was connected to an inert carrier gas to facilitate the flow of feedstock into the reactor and the discharge of products into the product collection module. The sealed pyrolysis reactor was enclosed inside a quartz tube with Swagelok fittings. After loading polyethylene into the 3D-printed reactor, an electrified heating program initiates pyrolysis and the products are collected in ethyl acetate or a cold trap for liquid products while gaseous products are collected in a gas bag in the collection module. Once the reaction is completed, both the heating and gas flow are stopped. b, An enlarged view of the pyrolysis reactor shows that it is sealed within the quartz tube and connected to both electrical power supply and inert carrier gas.
Extended Data Fig. 4 Surface temperature measurement of the graded-pore carbon reactor.
a, The surface temperature of the carbon pyrolysis reactor was measured using the optical two-color pyrometry method, which is based on Planck’s radiation law16. This method calculates temperature by analyzing radiation intensity at two wavelengths (650 nm and 750 nm), captured by a high-speed CMOS camera through bandpass filters. The camera’s measurements are synchronized and processed using a calibrated instrumental factor to determine the surface temperature. b, The temperature measurements of the three reactor zones over time clearly illustrate periodic fluctuations in surface temperature, as observed in the 3D graph. Additionally, the graph reveals a distinct temperature gradient across the three pyrolysis zones, indicating that each zone experiences a different temperature range, which is critical for optimizing the pyrolysis process and ensuring efficient heat distribution throughout the reactor. c, Characterization of the surface temperature at different time points. At the end of the 110 ms heating period, the temperature in the reactor reaches its maximum, with average peak temperatures in the three zones recorded as 630 °C, 525 °C, and 445 °C, respectively. Following 990 ms of cooling, the average surface temperatures decrease to 360 °C, 350 °C, and 330 °C across the same zones.
Extended Data Fig. 5 Interior temperature measurements of the graded-pore pyrolysis reactor using SFBGs.
The internal temperature of the pyrolysis reactor was measured using Femtosecond laser-inscribed single crystal sapphire fiber Bragg gratings (SFBGs). These precise temperature sensors were inscribed on a 4-inch sapphire optical fiber using a Femtosecond laser microfabrication system and placed in the reactor to monitor temperature during pulsed heating in an argon-controlled environment. The setup included a supercontinuum light source, multimode scrambler, Bayspec interrogator, and a glass-to-sapphire splice for light transmission. The SFBGs responded to temperature fluctuations by altering their refractive index, and the resulting wavelength shifts were detected and converted to temperature readings. Measurements across three reactor zones showed a clear temperature gradient: Zone 1 ranged from 630 °C to 530 °C, Zone 2 from 530 °C to 470 °C, and Zone 3 from 490 °C to 420 °C, as illustrated in Fig. 2d.
Extended Data Fig. 6 Sampling and analysis results of the liquid products from the LDPE pyrolysis reaction at different time intervals.
Following the initiation of the pyrolysis reaction in the graded-pore reactor, samples were collected at various time intervals and analyzed using GC-MS. To meet analysis requirements, the samples were diluted to a concentration of approximately 1–2 mg/mL (ethyl acetate used as the solvent, peak time = 2.4 min). No products were detected in the first 0–5 seconds. Subsequent analysis of the 5–10 second sample revealed the presence of hydrocarbons, indicating that product started to be produced during this period. Sampling was then performed every 10 seconds, showing similar product distributions from 10 to 80 seconds. Finally, the 80–90 second sample showed no detectable product peaks, suggesting the product concentration was below the detection limit and the reaction was completed during this time frame.
Extended Data Fig. 7 SEM observation of intermediates within the pore surfaces of the three reactor zones at different time points (20–90 s) during the LDPE pyrolysis.
a–d, Ex situ SEM imaging was conducted at different time points to observe the morphological changes of intermediates at different zones of the reactor over the course of the reaction. Four key time points—20 s (a), 40 s (b), 60 s (c), and 90 s (d)—were selected to capture the process. At 20 seconds, in Zone 1 (with a large pore size of 1.0 mm), the inner surface of the pores was covered with a melted coating. In Zone 2, scattered intermediate-sized coatings were visible, while no particles were detected in Zone 3. Given that reaction products were detected within 5–10 seconds of starting the reactor (Supplementary Fig. 7), this absence of particles in Zone 3 strongly suggests that intermediates have already undergone sufficient pyrolysis and vaporized in this zone, rather than simply not arriving there yet. A similar trend was observed at 40 and 60 seconds, with a more extensive coating in Zone 1 and larger intermediate coatings in Zone 2 at the 40-second mark. By 90 seconds, no intermediates were visible in any of the reactor zones, indicating the reaction was nearly complete, consistent with kinetic monitoring of liquid product formation over time (Supplementary Fig. 7).
Extended Data Fig. 8 Concentration profiles of pyrolysis intermediates with different molar mass at t = 8 s, t = 19 s, and t = 36 s, obtained through COMSOL Multiphysics simulation, demonstrating the gating effect of the graded-pore reactor on higher molar mass intermediates.
Transient concentration profiles of pyrolysis intermediates with different molar mass obtained through COMSOL Multiphysics simulations. We constructed a model of the graded-pore reactor featuring the same dimensions and porosity as the experimental reactor and simulated the progression of different molar mass intermediate hydrocarbons (C1532, C699, C111, C31, and C11) under the reaction conditions (see Methods; detailed concentration profiles throughout the pyrolysis process can be found in Video 1). The concentration profiles of the pyrolysis intermediates demonstrate the graded-pore reactor effectively regulates the mass transport of intermediates based on their molar mass. For example, at t = 8 s into the simulation, higher molar mass intermediates, such as C1532, remain primarily in Zone 1 (larger pore, higher-temperature). By t = 36 s, the C1532 species can be found in Zone 2, but are most concentrated at the boundary between Zones 2 and 3, suggesting the decrease in pore size between these zones slows the molecular species down from progressing through the reactor, effectively ‘gating’ the oligomer and increasing its residence time at that heating zone. Higher residence time under the heating conditions could promote further pyrolysis into lower molar mass intermediates, which are able to travel through the reactor more quickly. For example, the concentration profile of C11 at t = 8.0 s shows this lower molar mass intermediate is already present in Zone 3 and exiting the reactor, which could also help prevent over-pyrolysis into smaller gaseous molecules. We refer to this molecular-weight-based mass transport regulation mechanism as the "gating effect."
Extended Data Fig. 9 Large-scale experiments achieved using a gradient porosity carbon felt reactor.
a, To expand the design and applicability of the graded-pore reactor, a reaction apparatus was constructed using commercially available carbon felt materials. b, Similar to the previously constructed 3D-printed reactor, three different porosity carbon felts were placed inside a quartz tube in the pyrolysis reaction zones, with porosities of 94.0%, 91.3%, and 66.4% in Zones 1, 2, and 3, respectively. In the lower part of Zone 1, 20 g of LDPE feedstock was wrapped in carbon felt. The reaction apparatus was connected to an inert carrier gas flow (200 sccm) and power input (80–100 V), with a programmable controller preset to a pulsed heating cycle of 110 ms heating and 990 ms cooling. The inert carrier gas introduced at the bottom of the reaction tube drives the LDPE feedstock through the pyrolysis reactor. After passing through the reactor, the pyrolysis products are collected by a condenser immersed in a dry ice-ethanol bath. Meanwhile, any portion of the liquid hydrocarbon products that remained inside the pyrolysis reactor (that is, did not exit with the carrier gas) were also collected. This process was repeated 10 times to convert a total of 200 g of feedstock.
Extended Data Fig. 10 Combustion test: Adiabatic flame temperature.
a, The adiabatic flame temperature of the synthesized jet fuel was measured using the two-color pyrometry method, which assumes that the temperature of soot particle intermediates captured on camera is representative of the flame with negligible temperature difference. The setup, illustrated in Supplementary Fig. 26a, consisted of a Bunsen burner and an ICCD camera (Princeton Instruments PIMAX 1300). Operating under stoichiometric conditions at an initial temperature of 470 K, the flame was imaged twice using bandpass filters with center wavelengths of 650 nm and 750 nm. Although heat loss occurred during the experiment, the adiabatic flame temperature was approximated by selecting the maximum flame temperature where heat loss was minimized, with an estimated error of ~7%. b, Comparison of the adiabatic flame temperature with other common jet fuels. In the figure, jet fuel types A1, A2, A3, C1, C2, C3, C4, C5, and C6 represent typical jet fuels tested by the National Jet Fuels Combustion Program, each possessing specific combustion characteristics suited for different aviation applications17,18,19. When comparing the adiabatic flame temperatures of our synthesized fuel with those of these conventional jet fuels, we found that our jet fuel exhibits a higher adiabatic flame temperature of 2201 °C.
Supplementary information
Supplementary Information
Supplementary Figs. 1–19, Tables 1–20, Equations 1–27, Methods and References.
Supplementary Video 1
Simulation of the concentration profiles of different molar mass intermediate hydrocarbons (C1532, C699, C111, C31 and C11) throughout the pyrolysis process in a graded-pore reactor.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 10
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, J., Dong, Q., Zhang, C. et al. Selective electrified polyethylene upcycling by pore-modulated pyrolysis. Nat Chem Eng 2, 424–435 (2025). https://doi.org/10.1038/s44286-025-00248-0
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s44286-025-00248-0
This article is cited by
-
Joule-heating reactor powers scalable plastic upcycling
Nature Chemical Engineering (2025)