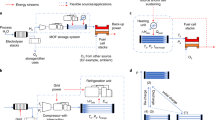
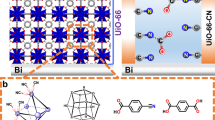
Abstract
Renewable electricity-driven water splitting is essential for decarbonizing high-emission industries and transportation. Metal–organic frameworks (MOFs) have shown great promise as catalytic materials for water splitting, but substantial gaps remain between fundamental research and practical application. Here we report the scalable and rapid synthesis of CoCe MOFs for alkaline water-splitting electrolyzers, achieving low energy consumption (4.11 kWh Nm−3 H2) and long-term stability (5,000 h). Experiments indicate that the advantageous physiochemical properties of CoCe MOFs such as lattice distortion and large specific surface area enhance catalytic activity, facilitate water and gas transport and improve electrolyte accessibility to catalytic interfaces in practical devices. Preliminary techno-economic analysis shows that the cost of hydrogen produced from the CoCe MOF-based electrolyzer is US$2.71 kg−1, which is close to the target cost set by the US Department of Energy, and a life cycle assessment indicates that green hydrogen has up to 84.5% lower life cycle carbon emissions than traditional gray hydrogen production pathways.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data needed to evaluate the conclusions in the paper are present in the paper and/or Supplementary Information. Source data are provided with this paper, including the atomic coordinates of the optimized computational models. Additional data related to this paper may be requested from the authors.
References
Liang, Q. et al. Efficient osmosis-powered production of green hydrogen. Nat. Sustain. 7, 628–639 (2024).
Guan, D. et al. Hydrogen society: from present to future. Energy Environ. Sci. 16, 4926–4943 (2023).
Turner, J. A. Sustainable hydrogen production. Science 305, 972–974 (2004).
Wang, P. et al. Urine electrooxidation for energy–saving hydrogen generation. Nat. Commun. 16, 2424 (2025).
Guo, J. et al. Direct seawater electrolysis by adjusting the local reaction environment of a catalyst. Nat. Energy 8, 264–272 (2023).
Do, T. N. et al. Carbon-neutral hydrogen production from natural gas via electrified steam reforming: techno-economic-environmental perspective. Energy Convers. Manage. 279, 116758 (2023).
Mahmud, R., Moni, S. M., High, K. & Carbajales-Dale, M. Integration of techno-economic analysis and life cycle assessment for sustainable process design–a review. J. Clean. Prod. 317, 128247 (2021).
Gholkar, P., Shastri, Y. & Tanksale, A. Renewable hydrogen and methane production from microalgae: a techno-economic and life cycle assessment study. J. Clean. Prod. 279, 123726 (2021).
Fu, R., Kang, L., Zhang, C. & Fei, Q. Application and progress of techno-economic analysis and life cycle assessment in biomanufacturing of fuels and chemicals. Green Chem. Eng. 4, 189–198 (2023).
Megía, P. J., Vizcaíno, A. J., Calles, J. A. & Carrero, A. Hydrogen production technologies: from fossil fuels toward renewable sources. A mini review. Energy Fuels 35, 16403–16415 (2021).
Dufour, J. et al. Life cycle assessment of alternatives for hydrogen production from renewable and fossil sources. Int. J. Hydrogen Energy 37, 1173–1183 (2012).
Nowotny, J. & Veziroglu, T. N. Impact of hydrogen on the environment. Int. J. Hydrogen Energy 36, 13218–13224 (2011).
Palmer, G., Roberts, A., Hoadley, A., Dargaville, R. & Honnery, D. Life-cycle greenhouse gas emissions and net energy assessment of large-scale hydrogen production via electrolysis and solar PV. Energy Environ. Sci. 14, 5113–5131 (2021).
Kwasi-Effah, C. C., Obanor, A. I. & Aisien, F. A. A review on electrolytic method of hydrogen production from water. J. Renewable Sustainable Energy 1, 51–57 (2015).
Brauns, J. & Turek, T. Alkaline water electrolysis powered by renewable energy: a review. Processes 8, 248 (2020).
Panigrahy, B., Narayan, K. & Rao, B. R. Green hydrogen production by water electrolysis: a renewable energy perspective. Mater. Today Proc. 67, 1310–1314 (2022).
Zhou, S., Shi, L., Li, Y., Yang, T. & Zhao, S. Metal‐organic framework‐based electrocatalysts for acidic water splitting. Adv. Funct. Mater. 34, 2400767 (2024).
Yu, F., Bai, X., Liang, M. & Ma, J. Recent progress on metal-organic framework-derived porous carbon and its composite for pollutant adsorption from liquid phase. Chem. Eng. J. 405, 126960 (2021).
Qian, Y., Zhang, F., Kang, D. J. & Pang, H. A review of metal–organic framework‐based compounds for environmental applications. Energy Environ. Mater. 6, e12414 (2023).
Zhao, S. et al. Ultrathin metal–organic framework nanosheets for electrocatalytic oxygen evolution. Nat. Energy 1, 16184 (2016).
Sun, Y. et al. Modulating electronic structure of metal-organic frameworks by introducing atomically dispersed Ru for efficient hydrogen evolution. Nat. Commun. 12, 1369 (2021).
Jiang, Y. et al. Heterostructured bimetallic MOF‐on‐MOF architectures for efficient oxygen evolution reaction. Adv. Mater. 36, 2306910 (2024).
Zhang, B. et al. Designing MOF nanoarchitectures for electrochemical water splitting. Adv. Mater. 33, 2006042 (2021).
Guo, Y. et al. Non-derivatized metal-organic framework nanosheets for water electrolysis: fundamentals, regulation strategies and recent advances. Chin. J. Catal. 67, 21–53 (2024).
Chen, C. et al. Local reaction environment in electrocatalysis. Chem. Soc. Rev. 53, 2022–2055 (2024).
Yang, H. et al. Potential-driven structural distortion in cobalt phthalocyanine for electrocatalytic CO2/CO reduction towards methanol. Nat. Commun. 15, 7703 (2024).
Zhao, S. et al. Structural transformation of highly active metal–organic framework electrocatalysts during the oxygen evolution reaction. Nat. Energy 5, 881–890 (2020).
Lyu, S. et al. Exceptional catalytic activity of oxygen evolution reaction via two-dimensional graphene multilayer confined metal-organic frameworks. Nat. Commun. 13, 6171 (2022).
Cheng, W. et al. Lattice-strained metal–organic-framework arrays for bifunctional oxygen electrocatalysis. Nat. Energy 4, 115–122 (2019).
Wu, W. et al. Structural transformation of metal–organic framework with constructed tetravalent nickel sites for efficient water oxidation. J. Energy Chem. 74, 404–411 (2022).
Hu, Y. et al. Modulating 3d charge state via halogen ions in neighboring molecules of metal–organic frameworks for improving water oxidation. Small 20, 2400042 (2024).
Wang, X. et al. Embedding oxophilic rare-earth single atom in platinum nanoclusters for efficient hydrogen electro-oxidation. Nat. Commun. 14, 3767 (2023).
Li, L. et al. Lanthanide-regulating Ru-O covalency optimizes acidic oxygen evolution electrocatalysis. Nat. Commun. 15, 4974 (2024).
Feng, J. et al. CO2 electrolysis to multi-carbon products in strong acid at ampere-current levels on La-Cu spheres with channels. Nat. Commun. 15, 4821 (2024).
Chen, L. et al. Energy-efficient CO2 conversion to multicarbon products at high rates on CuGa bimetallic catalyst. Nat. Commun. 15, 7053 (2024).
Yang, J. et al. Formation of hydrided Pt-Ce-H sites in efficient, selective oxidation catalysts. Science 388, 514–519 (2025).
Liu, X., Liu, T., Ouyang, T., Deng, J. & Liu, Z. Ce3+/Ce4+ Ion redox shuttle stabilized Cuδ+ for efficient CO2 electroreduction to C2H4. Angew. Chem. Int. Ed. 64, e202419796 (2025).
Yin, L. et al. Heteroatom‐driven coordination fields altering single cerium atom sites for efficient oxygen reduction reaction. Adv. Mater. 35, 2302485 (2023).
Wang, T. et al. Efficient C (sp3)–H bond oxidation on perovskite quantum dots based on Ce‐oxygen affinity. Angew. Chem. Int. Ed. 63, e202409656 (2024).
Wan, R. et al. Earth-abundant electrocatalysts for acidic oxygen evolution. Nat. Catal. 7, 1288–1304 (2024).
Roger, I., Shipman, M. & Symes, M. Earth-abundant catalysts for electrochemical and photoelectrochemical water splitting. Nat. Rev. Chem. 1, 0003 (2017).
Wong-Ng, W., Kaduk, J. A., Wu, H. & Suchomel, M. Synchrotron X-ray studies of metal-organic framework M2 (2, 5-dihydroxyterephthalate), M=(Mn, Co, Ni, Zn)(MOF-74). Powder Diffr. 27, 256–262 (2012).
Jun, H., Oh, S., Lee, G. & Oh, M. Enhanced catalytic activity of MOF-74 via providing additional open metal sites for cyanosilylation of aldehydes. Sci. Rep. 12, 14735 (2022).
Chen, C. et al. Microwave-assisted rapid synthesis of well-shaped MOF-74 (Ni) for CO2 efficient capture. Inorg. Chem. 58, 2717–2728 (2019).
Wang, X. et al. Engineering 3d–2p–4f gradient orbital coupling to enhance electrocatalytic oxygen reduction. Adv. Mater. 34, 2206540 (2022).
Li, M. et al. Reinforcing Co–O covalency via Ce(4f)–O(2p)–Co(3d) gradient orbital coupling for high‐efficiency oxygen evolution. Adv. Mater. 35, 2302462 (2023).
Guo, Z. et al. Manipulating the spin state of spinel octahedral sites via a π–π type orbital coupling to boost water oxidation. Angew. Chem. Int. Ed. 63, e202406711 (2024).
Hu, J. et al. Charge‐transfer‐regulated bimetal ferrocene‐based organic frameworks for promoting electrocatalytic oxygen evolution. Carbon Energy 5, e315 (2023).
Luo, S. et al. Optimizing alkaline water electrolysis: a dual-model approach for enhanced hydrogen production efficiency. Energies 17, 5512 (2024).
Montoya, J. H. et al. Materials for solar fuels and chemicals. Nat. Mater. 16, 70–81 (2017).
Luo, X. et al. Fe-S dually modulated adsorbate evolution and lattice oxygen compatible mechanism for water oxidation. Nat. Commun. 15, 8293 (2024).
Zhang, T. et al. Atomically thin high-entropy oxides via naked metal ion self-assembly for proton exchange membrane electrolysis. Nat. Commun. 16, 1037 (2025).
Hu, Y. et al. Understanding the sulphur-oxygen exchange process of metal sulphides prior to oxygen evolution reaction. Nat. Commun. 14, 1949 (2023).
Acknowledgements
S. Zhao is grateful for the support from the National Natural Science Foundation of China (grant no. 22373027). L.S. is grateful for the support from the National Natural Science Foundation of China (grant no. 22409199). We thank the 1W1B-XAFS and 1W2B-WAXS Beamline of Beijing Synchrotron Radiation Facility for providing technical support and assistance in data collection. We also thank J. Dong and P. An from Beijing Synchrotron Radiation Facility for XAFS data analysis. We are grateful for the support from Intelligent Environment Research Center in the LCA evaluation using SimaPro.
Author information
Authors and Affiliations
Contributions
S. Zhao proposed the research direction and supervised the project. Y.G. designed and performed the experiments. L.S. contributed to the theoretical calculation and experimental analysis. X.S. and S. Zhang supported the LCA and TEA. T.Z., W.T. and Y.L. assisted with parts of the experiments and data analysis. S. Zhao, F.Z. and D.L. contributed to theoretical and model development and supported data analysis. Y.G., L.S. and S. Zhao discussed the results and cowrote the manuscript. All authors participated in the discussion and the preparation of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemical Engineering thanks Shaojun Guo, Lei Wang, Yao Zheng and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary discussions, Figs. 1–53 and Tables 1–14.
Supplementary Video 1
Supplementary Video 1
Supplementary Video 2
Supplementary Video 2
Supplementary Data 1
Atomic coordinates of the optimized computational models.
Source data
Source Data Fig. 1
Source data for Fig. 1.
Source Data Fig. 2
Source data for Fig. 2.
Source Data Fig. 3
Source data for Fig. 3.
Source Data Fig. 4
Source data for Fig. 4.
Source Data Fig. 5
Source data for Fig. 5.
Source Data Fig. 6
Source data for Fig. 6.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guo, Y., Shi, L., Shi, X. et al. Scalable metal–organic framework-based electrodes for efficient alkaline water electrolysis. Nat Chem Eng 2, 474–483 (2025). https://doi.org/10.1038/s44286-025-00262-2
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s44286-025-00262-2
This article is cited by
-
Scaling up metal-organic frameworks for efficient kilowatt-level alkaline water electrolysis
Science China Chemistry (2026)
-
Metal–organic-framework-based electrolyzers with mass appeal
Nature Chemical Engineering (2025)
-
Sustainable ammonia production beyond Haber-Bosch: a review of nitrogen reduction pathways from diverse feedstocks
Science China Chemistry (2025)