Abstract
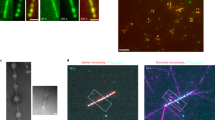
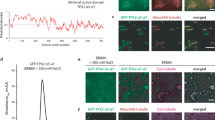
The self-organization of cytoskeletal biopolymers, such as microtubules (MTs), depends on mechanosensing and adaptation to confined spaces such as cellular protrusions. Understanding how these active biopolymers coordinate their formation under confinement leads to advances in bioengineering. Here we report the self-organization of branched MT networks in channels with narrow junctions and closed ends, mimicking cellular protrusions. We find that branching MT nucleation occurs in the post-narrowing region only if this region exceeds a minimum length, determined by MT dynamic instability at the closed end and the timescale for nucleation at a distant point. We term this feedback ‘boundary sensing’. Increasing the amount of branching factor TPX2 in the system accelerates MT nucleation and adjusts this minimum length, but excess TPX2 stabilizes MTs at the closed end, disrupting network formation. We performed experiments and simulations to study how this tunable feedback, wherein growing MTs navigate confinement and create nucleation sites, shapes MT architecture. Our findings impact the understanding of MT self-organization during axonal growth, dendrite formation, plant development, fungal guidance and the engineering of biomaterials.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this Article and its Supplementary Information files.
Code availability
The MATLAB codes used for simulations performed in this study are included in the Supplementary Information files.
References
Miyazaki, M., Chiba, M., Eguchi, H., Ohki, T. & Ishiwata, S. Cell-sized spherical confinement induces the spontaneous formation of contractile actomyosin rings in vitro. Nat. Cell Biol. 17, 480–489 (2015).
Bashirzadeh, Y., Wubshet, N. H. & Liu, A. P. Confinement geometry tunes fascin-actin bundle structures and consequently the shape of a lipid bilayer vesicle. Front. Mol. Biosci. 7, 610277 (2020).
Gai, Y., Cook, B., Setru, S., Stone, H. A. & Petry, S. Confinement size determines the architecture of Ran-induced microtubule networks. Soft Matter 17, 5921–5931 (2021).
Pinot, M. et al. Effects of confinement on the self-organization of microtubules and motors. Curr. Biol. 19, 954–960 (2009).
Oakes, P. W., Banerjee, S., Marchetti, M. C. & Gardel, M. L. Geometry regulates traction stresses in adherent cells. Biophys. J. 107, 825–833 (2014).
Araújo, N. A. et al. Steering self-organisation through confinement. Soft Matter 19, 1695–1704 (2023).
Swanson, D. & Wingreen, N. S. Active biopolymers confer fast reorganization kinetics. Phys. Rev. Lett. 107, 218103 (2011).
Andreu, I. et al. The force loading rate drives cell mechanosensing through both reinforcement and cytoskeletal softening. Nat. Commun. 12, 4229 (2021).
Seetharaman, S. et al. Microtubules tune mechanosensitive cell responses. Nat. Mater. 21, 366–377 (2022).
Clarke, D. N. & Martin, A. C. Actin-based force generation and cell adhesion in tissue morphogenesis. Curr. Biol. 31, R667–R680 (2021).
Jiang, X., Bruzewicz, D. A., Wong, A. P., Piel, M. & Whitesides, G. M. Directing cell migration with asymmetric micropatterns. Proc. Natl Acad. Sci. USA 102, 975–978 (2005).
Mahmud, G. et al. Directing cell motions on micropatterned ratchets. Nat. Phys. 5, 606–612 (2009).
Vignaud, T., Blanchoin, L. & Théry, M. Directed cytoskeleton self-organization. Trends Cell Biol. 22, 671–682 (2012).
Théry, M. Micropatterning as a tool to decipher cell morphogenesis and functions. J. Cell Sci. 123, 4201–4213 (2010).
Han, B. et al. Conversion of mechanical force into biochemical signaling. J. Biol. Chem. 279, 54793–54801 (2004).
Gardel, M. L., Kasza, K. E., Brangwynne, C. P., Liu, J. & Weitz, D. A. Mechanical response of cytoskeletal networks. Methods Cell Biol. 89, 487–519 (2008).
Sakamoto, R. et al. Tug-of-war between actomyosin-driven antagonistic forces determines the positioning symmetry in cell-sized confinement. Nat. Commun. 11, 3063 (2020).
Banerjee, S., Gardel, M. L. & Schwarz, U. S. The actin cytoskeleton as an active adaptive material. Annu. Rev. Condens. Matter Phys. 11, 421–439 (2020).
Amiri, S. et al. Intracellular tension sensor reveals mechanical anisotropy of the actin cytoskeleton. Nat. Commun. 14, 8011 (2023).
Sakamoto, R., Izri, Z., Shimamoto, Y., Miyazaki, M. & Maeda, Y. T. Geometric trade-off between contractile force and viscous drag determines the actomyosin-based motility of a cell-sized droplet. Proc. Natl Acad. Sci. USA 119, e2121147119 (2022).
Stachowiak, M. R. et al. A mechanical-biochemical feedback loop regulates remodeling in the actin cytoskeleton. Proc. Natl Acad. Sci. USA 111, 17528–17533 (2014).
Risca, V. I. et al. Actin filament curvature biases branching direction. Proc. Natl Acad. Sci. USA 109, 2913–2918 (2012).
Dogterom, M. & Koenderink, G. H. Actin–microtubule crosstalk in cell biology. Nat. Rev. Mol. Cell Biol. 20, 38–54 (2019).
Stehbens, S. J. et al. CLASPs link focal-adhesion-associated microtubule capture to localized exocytosis and adhesion site turnover. Nat. Cell Biol. 16, 558–570 (2014).
Gierke, S. & Wittmann, T. EB1-recruited microtubule +TIP complexes coordinate protrusion dynamics during 3D epithelial remodeling. Curr. Biol. 22, 753–762 (2012).
Schmidt, C. J. & Stehbens, S. J. Microtubule control of migration: coordination in confinement. Curr. Opin. Cell Biol. 86, 102289 (2024).
Lavrsen, K. et al. Microtubule detyrosination drives symmetry breaking to polarize cells for directed cell migration. Proc. Natl Acad. Sci. USA 120, e2300322120 (2023).
Brangwynne, C. P. et al. Microtubules can bear enhanced compressive loads in living cells because of lateral reinforcement. J. Cell Biol. 173, 733–741 (2006).
Li, Y. et al. Compressive forces stabilize microtubules in living cells. Nat. Mater. 22, 913–924 (2023).
Brouhard, G. J. & Rice, L. M. Microtubule dynamics: an interplay of biochemistry and mechanics. Nat. Rev. Mol. Cell Biol. 19, 451–463 (2018).
Schaedel, L. et al. Microtubules self-repair in response to mechanical stress. Nat. Mater. 14, 1156–1163 (2015).
Ju, R. J. et al. Compression-dependent microtubule reinforcement enables cells to navigate confined environments. Nat. Cell Biol. 26, 1520–1534 (2024).
Ndlec, F., Surrey, T., Maggs, A. C. & Leibler, S. Self-organization of microtubules and motors. Nature 389, 305–308 (1997).
Sulerud, T. et al. Microtubule-dependent pushing forces contribute to long-distance aster movement and centration in Xenopus laevis egg extracts. Mol. Biol. Cell 31, 2791–2802 (2020).
Holy, T. E., Dogterom, M., Yurke, B. & Leibler, S. Assembly and positioning of microtubule asters in microfabricated chambers. Proc. Natl Acad. Sci. USA 94, 6228–6231 (1997).
Suzuki, K., Miyazaki, M., Takagi, J., Itabashi, T. & Ishiwata, S. Spatial confinement of active microtubule networks induces large-scale rotational cytoplasmic flow. Proc. Natl Acad. Sci. USA 114, 2922–2927 (2017).
Inoue, D. et al. Adaptation of patterns of motile filaments under dynamic boundary conditions. ACS Nano 13, 12452–12460 (2019).
López, M. P. et al. Actin–microtubule coordination at growing microtubule ends. Nat. Commun. 5, 4778 (2014).
Théry, M. et al. Anisotropy of cell adhesive microenvironment governs cell internal organization and orientation of polarity. Proc. Natl Acad. Sci. USA 103, 19771–19776 (2006).
Gélin, M. et al. Microtubules under mechanical pressure can breach dense actin networks. J. Cell Sci. 136, jcs261667 (2023).
Yamamoto, S. et al. Actin network architecture can ensure robust centering or sensitive decentering of the centrosome. EMBO J. 41, e111631 (2022).
Murata, T. et al. Microtubule-dependent microtubule nucleation based on recruitment of γ-tubulin in higher plants. Nat. Cell Biol. 7, 961–968 (2005).
Nakamura, M., Ehrhardt, D. W. & Hashimoto, T. Microtubule and katanin-dependent dynamics of microtubule nucleation complexes in the acentrosomal Arabidopsis cortical array. Nat. Cell Biol. 12, 1064–1070 (2010).
Jacobs, B., Schneider, R., Molenaar, J., Filion, L. & Deinum, E. E. Microtubule nucleation complex behavior is critical for cortical array homogeneity and xylem wall patterning. Proc. Natl Acad. Sci. USA 119, e2203900119 (2022).
Mirabet, V. et al. The self-organization of plant microtubules inside the cell volume yields their cortical localization, stable alignment, and sensitivity to external cues. PLoS Comput. Biol. 14, e1006011 (2018).
Shi, X. & Ma, Y. Understanding phase behavior of plant cell cortex microtubule organization. Proc. Natl Acad. Sci. USA 107, 11709–11714 (2010).
Yan, Y., Sun, Z., Yan, P., Wang, T. & Zhang, Y. Mechanical regulation of cortical microtubules in plant cells. New Phytol. 239, 1609–1621 (2023).
Hejnowicz, Z., Rusin, A. & Rusin, T. Tensile tissue stress affects the orientation of cortical microtubules in the epidermis of sunflower hypocotyl. J. Plant Growth Regul. 19, 31–44 (2000).
Hamant, O., Inoue, D., Bouchez, D., Dumais, J. & Mjolsness, E. Are microtubules tension sensors? Nat. Commun. 10, 2360 (2019).
Petry, S., Groen, A. C., Ishihara, K., Mitchison, T. J. & Vale, R. D. Branching microtubule nucleation in Xenopus egg extracts mediated by augmin and TPX2. Cell 152, 768–777 (2013).
Petry, S. Mechanisms of mitotic spindle assembly. Annu. Rev. Biochem. 85, 659–683 (2016).
Decker, F., Oriola, D., Dalton, B. & Brugues, J. Autocatalytic microtubule nucleation determines the size and mass of Xenopus laevis egg extract spindles. Elife 7, e31149 (2018).
Kraus, J., Travis, S. M., King, M. R. & Petry, S. Augmin is a Ran-regulated spindle assembly factor. J. Biol. Chem. 299, 104736 (2023).
Travis, S. M. et al. Integrated model of the vertebrate augmin complex. Nat. Commun. 14, 2072 (2023).
Guo, C. et al. Structural basis of protein condensation on microtubules underlying branching microtubule nucleation. Nat. Commun. 14, 3682 (2023).
Gouveia, B. et al. Acentrosomal spindles assemble from branching microtubule nucleation near chromosomes in Xenopus laevis egg extract. Nat. Commun. 14, 3696 (2023).
Paredez, A. R., Somerville, C. R. & Ehrhardt, D. W. Visualization of cellulose synthase demonstrates functional association with microtubules. Science 312, 1491–1495 (2006).
Sasaki, T. et al. Confined-microtubule assembly shapes three-dimensional cell wall structures in xylem vessels. Nat. Commun. 14, 6987 (2023).
Higa, T. et al. Microtubule-associated phase separation of MIDD1 tunes cell wall spacing in xylem vessels in Arabidopsis thaliana. Nat. Plants 10, 100–117 (2024).
Stiess, M. et al. Axon extension occurs independently of centrosomal microtubule nucleation. Science 327, 704–707 (2010).
Sánchez-Huertas, C. et al. Non-centrosomal nucleation mediated by augmin organizes microtubules in post-mitotic neurons and controls axonal microtubule polarity. Nat. Commun. 7, 12187 (2016).
Zhang, Y. et al. Augmin complex activity finetunes dendrite morphology through non-centrosomal microtubule nucleation in vivo. J. Cell Sci. 137, jcs261512 (2024).
Mukherjee, A. et al. γ-TuRCs and the augmin complex are required for the development of highly branched dendritic arbors in Drosophila. J. Cell Sci. 137, jcs261534 (2024).
Zaferani, M., Song, R., Petry, S. & Stone, H. A. Building on-chip cytoskeletal circuits via branched microtubule networks. Proc. Natl Acad. Sci. USA 121, e2315992121 (2024).
Thawani, A., Stone, H. A., Shaevitz, J. W. & Petry, S. Spatiotemporal organization of branched microtubule networks. Elife 8, e43890 (2019).
Pullarkat, P. A., Dommersnes, P., Fernández, P., Joanny, J.-F. & Ott, A. Osmotically driven shape transformations in axons. Phys. Rev. Lett. 96, 048104 (2006).
Andersson, M. et al. Axon morphology is modulated by the local environment and impacts the noninvasive investigation of its structure–function relationship. Proc. Natl Acad. Sci. USA 117, 33649–33659 (2020).
King, M. R. & Petry, S. Phase separation of TPX2 enhances and spatially coordinates microtubule nucleation. Nat. Commun. 11, 270 (2020).
Setru, S. U. et al. A hydrodynamic instability drives protein droplet formation on microtubules to nucleate branches. Nat. Phys. 17, 493–498 (2021).
Roostalu, J., Cade, N. I. & Surrey, T. Complementary activities of TPX2 and chTOG constitute an efficient importin-regulated microtubule nucleation module. Nat. Cell Biol. 17, 1422–1434 (2015).
Mimori-Kiyosue, Y. & Tsukita, S. “Search-and-capture” of microtubules through plus-end-binding proteins (+TIPs). J. Biochem. 134, 321–326 (2003).
Heald, R. & Khodjakov, A. Thirty years of search and capture: the complex simplicity of mitotic spindle assembly. J. Cell Biol. 211, 1103–1111 (2015).
Dema, A., Charafeddine, R., Rahgozar, S., van Haren, J. & Wittmann, T. Growth cone advance requires EB1 as revealed by genomic replacement with a light-sensitive variant. Elife 12, e84143 (2023).
Dema, A. et al. Doublecortin reinforces microtubules to promote growth cone advance in soft environments. Curr. Biol. 34, 5822–5832 (2024).
Held, M., Kašpar, O., Edwards, C. & Nicolau, D. V. Intracellular mechanisms of fungal space searching in microenvironments. Proc. Natl Acad. Sci. USA 116, 13543–13552 (2019).
Kwan, A. C., Dombeck, D. A. & Webb, W. W. Polarized microtubule arrays in apical dendrites and axons. Proc. Natl Acad. Sci. USA 105, 11370–11375 (2008).
Baas, P. W., Deitch, J. S., Black, M. M. & Banker, G. A. Polarity orientation of microtubules in hippocampal neurons: uniformity in the axon and nonuniformity in the dendrite. Proc. Natl Acad. Sci. USA 85, 8335–8339 (1988).
Gavriljuk, K. et al. A self-organized synthetic morphogenic liposome responds with shape changes to local light cues. Nat. Commun. 12, 1548 (2021).
Sato, Y., Hiratsuka, Y., Kawamata, I., Murata, S. & Nomura, S. M. Micrometer-sized molecular robot changes its shape in response to signal molecules. Sci. Robot. 2, eaal3735 (2017).
Kattan, J., Doerr, A., Dogterom, M. & Danelon, C. Shaping liposomes by cell-free expressed bacterial microtubules. ACS Synth. Biol. 10, 2447–2455 (2021).
Wang, S. & Urban, M. W. Self-healing polymers. Nat. Rev. Mater. 5, 562–583 (2020).
Petry, S., Pugieux, C., Nédélec, F. J. & Vale, R. D. Augmin promotes meiotic spindle formation and bipolarity in Xenopus egg extracts. Proc. Natl Acad. Sci. USA 108, 14473–14478 (2011).
Acknowledgements
M.Z. acknowledges an Omenn-Darling Postdoctoral Fellowship from the Princeton Bioengineering Institute. This work was supported by the Princeton University Eric and Wendy Schmidt Transformative Technology Fund.
Author information
Authors and Affiliations
Contributions
M.Z., N.S.W., H.A.S. and S.P. conceived the project. M.Z. and R.S. conducted the experiments, data analysis and simulations. M.Z., N.S.W. and H.A.S. developed the model. H.A.S. and S.P. supervised the project. All authors contributed to the writing of the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemical Engineering thanks Yusuke T. Maeda and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Discussion and Figs. 1–4.
Supplementary Video 1
Self-organization of a branched microtubule (MT) network in front of a flat, rigid physical boundary. The average angle between the MT network and the wall is 85 ± 10°. MTs are visualized in magenta, and EB1 comets appear in green. Branching MT nucleation was induced by the addition of 10 µM RanQ69L.
Supplementary Video 2
Self-organization of a branched MT network within a microchannel containing a narrowing region and a closed end. Branching MT nucleation was induced by the addition of 10 µM RanQ69L.
Supplementary Video 3
Self-organization of a branched MT network within microchannels containing narrowing regions and post-narrowing regions of varying lengths. Branching MT nucleation was induced by the addition of 10 µM RanQ69L.
Supplementary Video 4
Growth of individual MTs within the narrowing region when it was empty (top) compared with growth when the narrowing region was pre-filled with 5–7 MTs (bottom). Branching MT nucleation was induced by the addition of 10 µM RanQ69L.
Supplementary Video 5
Self-organization of a branched MT network through a narrowing region approximately 400 nm in width. Branching MT nucleation was induced by the addition of 10 µM RanQ69L.
Supplementary Video 6
Self-organization of branched MT networks within a microchannel containing two narrowing and two post-narrowing regions. Branching MT nucleation was induced by the addition of 10 µM RanQ69L.
Supplementary Video 7
Self-organization of a branched MT network within microchannels containing narrowing regions and post-narrowing regions of length L = 9 µm in the presence of 10 µM RanQ69L and 350 nM TPX2. Branching MT nucleation was induced by the combined addition of RanQ69L and TPX2.
Supplementary Video 8
Self-organization of a branched MT network within microchannels containing narrowing regions and post-narrowing regions of lengths L = 5 µm and L = 15 µm in the presence of 10 µM RanQ69L and 1 µM TPX2. Branching MT nucleation was induced by the combined addition of RanQ69L and TPX2.
Supplementary Video 9
Computational simulations of branched MT network self-organization in a symmetric bifurcation with side channels of lengths L = 5 µm and L = 15 µm (top). Simulations of branched MT network self-organization in a symmetric bifurcation with higher TPX2 concentration in one side channel (bottom).
Supplementary Video 10
Self-organization of a branched MT network in a symmetric bifurcation with side channels of lengths L = 5 µm and L = 15 µm. Branching MT nucleation was induced by the addition of 10 µM RanQ69L.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zaferani, M., Song, R., Wingreen, N.S. et al. Boundary-sensing mechanism in branched microtubule networks. Nat Chem Eng 2, 498–510 (2025). https://doi.org/10.1038/s44286-025-00264-0
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s44286-025-00264-0
This article is cited by
-
A search-and-branch mechanism for microtubule sensing
Nature Chemical Engineering (2025)