Abstract
Placenta Accreta Spectrum (PAS) and pre-eclampsia are obstetric disorders associated with significant maternal morbidity and mortality. As pre-eclampsia is characterised by shallow placental invasion and PAS by abnormally deep invasion, we sought to compare the biological processes dysregulated in PAS to those reported in pre-eclampsia. We demonstrate that many of the processes contributing to unregulated placental invasion in PAS are the mirror findings of those previously reported in pre-eclampsia. These comparisons of PAS to a more common obstetric condition allow a greater understanding of the biological processes contributing to PAS, which remains a very rare obstetric complication.
Similar content being viewed by others
Background
The human placenta has three types of epithelial trophoblasts, namely cytotrophoblasts, syncytiotrophoblasts and extravillous Trophoblasts (EVT)1. The syncytiotrophoblast is a nucleated layer which forms the outer layer of the placenta2. This layer forms the interface between the foetal placenta and maternal blood and is essential for the exchange of nutrients, oxygen and waste products between the fetus and mother, as well as production of placental hormones such as human chorionic gonadotropin and progesterone2,3,4. Cytotrophoblasts form trophoblast cell columns, which undergo epithelial to mesenchymal transition (EMT) and gain invasive properties and are then referred to as EVT5. EVT, arising from the distal side of trophoblast cell columns, divide into two subpopulations, namely endovascular and interstitial EVT5,6. Endovascular EVT invade and remodel uterine spiral arteries, transforming them into low resistance vessels which supply blood into the intervillous space7,8. Interstitial EVT invade the decidual stroma and the superficial myometrium8.
EVT invasion into the decidua and myometrium are essential to establish normally placentation and maintain a healthy pregnancy. Placenta Accreta Spectrum (PAS) and early onset pre-eclampsia are two obstetric conditions where abnormal trophoblast invasion results in significant maternal morbidity and mortality9,10. PAS is a very rare disease, although the incidence of PAS is thought to be increasing due to the rise in the number of women giving birth by Caesarean section11,12. As more women and their families are likely to be impacted by PAS in line with the globally rising Caesarean delivery rate, a good understanding of the disease pathophysiology is essential in order to develop prevention strategies.
In PAS, following iatrogenic uterine injury from a prior surgery such as a Caesarean section, which results in myometrial scarring13, in a subsequent pregnancy placental implantation over this scarred area can result in abnormal placental attachment. There is ongoing debate and controversy as to the aetiology of PAS. This is reflected in the wide heterogeneity of cases described as PAS in the literature14, particularly relating to the condition placenta percreta15,16. While previously described as a condition of abnormal placental invasion, recent studies have proposed PAS results from iatrogenic myometrial and decidual damage, which allows the normally invasive placenta to abnormally attach to the myometrium9. As the placenta grows within this scarred myometrium this results in scar dehiscence, which may occur with or without abnormal adherence, resulting in the intraoperative findings classically described in PAS such placental bulge and distortion of the lower uterine segment17,18. While some would consider scar or uterine dehiscence as part of or a component of PAS aetiology, others suggest these cases warrant separate classification to reflect their different management approach18,19,20. Nonetheless, we and others suggest there are biological abnormalities at the maternal-foetal interface in PAS where an overly invasive and immunosuppressed local phenotype results in abnormal placental adherence, likely as a result of decidual damage and subsequent abnormal signalling21,22. PAS is an important obstetric complication as it is associated with significant maternal morbidity, generally related to massive obstetric haemorrhage23,24,25.
Conversely, pre-eclampsia, is a relatively common pregnancy complication affecting approximately 8% of pregnancies26,27. Pre-eclampsia has a varied clinical presentation, usually consisting of new-onset hypertension and proteinuria in pregnancy26,27. Risk factors for pre-eclampsia include hypertension, first time pregnancy, family or personal history of pre-eclampsia, and renal disease10,28,29. Early onset pre-eclampsia is characterised by a defective decidua which fails to correctly regulate EVT invasion, resulting in shallow trophoblast implantation and narrow maternal spiral artery formation30,31,32,33. This results in uteroplacental malperfusion and oxidative stress of the syncytiotrophoblast, leading to the release of various pro-inflammatory cytokines and anti-angiogenic markers into the maternal circulation34,35,36,37. Subsequently, local placental stress results in dysfunction of maternal endothelial cells and in systemic symptoms such as hypertension, proteinuria and intra-uterine foetal growth restriction30,31,32,33.
Therefore, in both PAS and pre-eclampsia there is abnormal placental invasion secondary to abnormalities of the decidua (Fig. 1). Comparisons between both conditions may provide important information regarding the underlying mechanisms of disease. We have shown that the biologic changes at the maternal-foetal interface seen in severe PAS represent an abnormal upregulation of the normal processes required for placental invasion, likely as a result of defective decidualisation22. As pre-eclampsia is characterised by abnormally shallow trophoblast invasion, we hypothesise that many of the biological processes dysregulated in PAS are differentially affected in pre-eclampsia. The extensive body of literature available for this relatively common condition could be used to further interrogate the pathophysiology of the much rarer condition, PAS. Here, we compare our previous findings of the pathophysiology of PAS22, to those reported in the literature in pre-eclampsia.
PAS is a uterine disorder secondary to iatrogenic uterine injury, which results in a defective or absent decidua, and deep myometrial EVT invasion. In pre-eclampsia, decidual resistance results in shallow trophoblast invasion, and subsequent systemic maternal and foetal complications. PAS Placenta Accreta Spectrum. EVT Extravillous Trophoblast.
Biological comparisons: PAS and pre-eclampsia
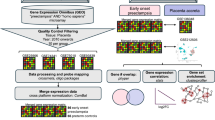
Using a multi-omic approach consisting of spatial transcriptomics and proteomics, we identified several new pathways which may contribute to the abnormal placental adherence in PAS22. These related to upregulation of genes and proteins involved in degradation of extracellular matrix (ECM), local immune suppression and increased cell adhesion and proliferation22. This supports the findings of other molecular PAS studies, which found trophoblasts cells are overly invasive38, altered immune balance at the maternal-foetal interface39 and the decidua defective resulting in abnormal placental attachment9,21. On the other hand, EVT invasion in pre-eclampsia is abnormally shallow, leading to systemic foetal and maternal complications. Comparing our findings from PAS cases, hallmarked by placental villi gaining deep access to the myometrium, to findings from pre-eclampsia studies we found many of the findings presented in our multi-omic study on PAS22 are the mirror image of those reported in the literature in pre-eclampsia (Fig. 2).
In a normal pregnancy, EVT gain invasive properties from CCT via EMT and divide into endovascular (transform spiral arteries into low resistance vessels) and interstitial EVTs (invade the decidua and superficial myometrium). In PAS, the decidua is absent or deficient, and the maternal myometrium is scarred and thinned. EVTs gain deep access to the myometrium in PAS, while pre-eclampsia is characterised by shallow EVT invasion. The figure shows findings seen in our study in severe PAS are often mirrored in pre-eclampsia22. In PAS, many transcription factors involved in normal trophoblast invasion are upregulated and these have previously been found to be downregulated in pre-eclampsia. Similarly, we found PAS regions expressed anti-apoptotic markers possibly resulting in increased cell-proliferation, while placentas in pre-eclampsia are pro-apoptotic. Immune changes seen at the maternal-foetal interface in PAS, such as increased PD-L1 and PD-L2, may allow trophoblast cells to escape the normal immune regulators of trophoblast invasion. Again, the mirror findings were previously reported in pre-eclampsia. Similarly, changes seen in the maternal serum in PAS, such as increased sTIE-2 and VEGF-A, the reverse findings were previously demonstrated in pre-eclampsia. vCTB villous cytotrophoblast. ST Syncytiotrophoblast. CCT cytotrophoblast column. EVT extravillous trophoblast. EMT epithelial to mesenchymal transition. ECM Extracellular Matrix.
Differentially expressed genes and proteins contributing to trophoblast invasion
Using spatial transcriptomics, we found several genes were differentially expressed in PAS. NF-κB2, expressed in syncytiotrophoblast and placental villi40, was increased in increta compared to accreta regions, which is part of the NF-κB protein family and increases EVT invasion by activating EMT41. Interestingly, in pre-eclampsia NF-κB2 is also significantly increased in placental tissue42. This may be explained as NF-κB can both inhibit or promote invasion via EMT. For example, when activated by TNF-alpha, NF-κB inhibits EVT invasion as a result of increased plasminogen activator-143, levels of which are increased in pre-eclampsia44. This suggests activation of NF-κB by different pathways in PAS and pre-eclampsia contributes to either increased or shallow EVT invasion. In PAS, this may be driven via PI3K/AKT/NF-κB45 and AP-1 signalling, which we showed were increased in increta regions22.
In another PAS spatial transcriptomics study by Afshar et al. 21, they identified several key differentially expressed genes in PAS including EGFL6, hepatocyte growth factor (HGF) and APOLD1. EGFL6, expressed in endothelial and decidual cells, was significantly increased in PAS, reflecting the findings of our PAS study21,22. EGFL6 is involved in growth promotion and angiogenesis46. Conversely, in pre-eclampsia, EGFL6 is reduced compared to healthy pregnancies47. Furthermore, Afshar et al.21 found HGF, another growth promoting gene expressed in endothelial decidual cells involved in normal trophoblast invasion, was one of the key genes differentially expressed in adherent placental regions in PAS. The reverse findings were previously demonstrated in pre-eclampsia, with placental production of HGF decreased, suggesting it has a possible role in the reduced trophoblast invasion seen in pre-eclampsia48. Similarly, APOLD1, which is expressed in endothelial cells and is involved in angiogenesis49, was significantly increased in adherent PAS regions and conversely is decreased in placentas in pre-eclampsia21,50. Therefore, while key genes involved in growth promotion and placental invasion appear overexpressed at the maternal-foetal interface in PAS21, in contrast these are downregulated in pre-eclampsia.
Additionally, we found several genes which were previously shown to be dysregulated in the decidua of pre-eclampsia such as ACTG2, CST3 and IGFBP351, which is involved in promotion of cell proliferation and growth52,exhibited the opposite expression pattern in PAS regions in our study22. For example, CST3 encodes cystatin C which is an inhibitor of cysteine proteinases and therefore inhibits trophoblast invasion, and is significantly upregulated in the decidua of placentas in pre-eclampsia51,53. In contrast, we found CST3 expression was reduced in PAS regions22. This suggests differential expression of these genes may contribute to the abnormal placental villi invasion in PAS and pre-eclampsia.
Dysregulated immune signalling leading to abnormal EVT invasion in PAS and pre-eclampsia
In a normal pregnancy, adaptations of the maternal immune system in response to the fetus play a critical role in ensuring pregnancy implantation, maintenance and completion of a healthy pregnancy54. EVT cells overexpress major histocompatibility (MHC) molecules HLA-C, HLA-G, and HLA-E to avoid attack by the maternal immune system55. PD-1 and its ligands are highly expressed by syncytiotrophoblast cells and increase as gestational age advances56. PD-1 and PD-L1 interaction with HLA-G expressing trophoblasts plays an important role in creating an immunosuppressed environment at the maternal-foetal interface to prevent foetal rejection57. This careful immune balance appears to be disrupted in both PAS and pre-eclampsia. In PAS, we and others, found that increta regions are characterised by local immune suppression at the maternal interface, as evidenced by reduced CD4 cells58, increased expression of PD-1 and its ligands PD-L1 and PD-L2, and increased expression of MHC molecules22.
In pre-eclampsia, there is dysregulation of the MHC class 1 molecules (HLA-G, HLA-C, HLA-F and HLA-E)55,59. HLA-G, which is only expressed on foetal trophoblasts and is particularly important for regulating maternal-foetal tolerance in normal pregnancies, is downregulated in pre-eclampsia59,60. This is thought to make EVTs in pre-eclampsia more vulnerable to the maternal immune system resulting in shallow invasion59,60. We showed increased HLA-G expression in PAS regions22, a possible hypothesis being EVTs in PAS may adapt to evade the maternal immune system to allow deeper invasion as a result of myometrial scarring. Furthermore, foetal trophoblasts expressing HLA-C C2 subtype in combination with maternal decidual natural killer cells lacking killer immunoglobulin receptors is associated with a significantly increased risk of pre-eclampsia61.
Moreover, while we showed PAS placentas had increased expression of PD-L1 and PD-L222, in pre-eclampsia there is reduced expression in placentas56,62,63. PD-L1 is expressed predominantly in syncytiotrophoblast and EVT cells64. In pre-eclampsia, decreased PD-L1 expression is thought to contribute to a dysfunctional immune balance at the maternal-foetal interface by promoting Th17 cell proliferation and inhibiting Treg cell differentiation62. Furthermore, PD-L1 negatively regulates migration and invasion of trophoblasts56.
These findings highlight the importance of immune signalling between the foetal and maternal cells at the maternal-foetal interface to establish and maintain normal placentation and pregnancy. In PAS and pre-eclampsia, dysregulated immune signalling is likely a key contributor to impaired EVT invasion. Importantly, in PAS these biological changes are likely secondary to myometrial injury21.
Apoptosis, cell proliferation and ECM degradation at the maternal-foetal interface contributing to abnormal EVT invasion
In PAS, there is abundance of anti-apoptotic proteins at the maternal foetal interface, such as BCL-2 and fibronectin, suggesting increased local cell proliferation and migration21,22,65. BCL-2 is an important regulator of trophoblast apoptosis in normal pregnancy66,67. Conversely, in pre-eclampsia, BCL-2 and other anti-apoptotic proteins are decreased68,69,70. These changes suggest a proliferative phenotype in PAS regions, in contrast to pre-eclampsia which is characterised by increased placental apoptosis and subsequently shallow EVT invasion68,71.
Interestingly, trophoblast apoptosis activates the complement pathway72, and we found on pathway analysis of differentially expressed genes, initial triggering of complement/complement cascade pathways were significantly downregulated in increta regions compared to accreta22. This is the opposite of what is reported in pre-eclampsia, where excessive placental complement deposition and pathway activation is seen in both the placenta and in maternal serum73,74,75,76. This association may partly be explained by the apoptotic placenta which is characteristic of pre-eclampsia72. The complement system is a key regulator of maternal-foetal tolerance77, further suggesting these biological changes in PAS and pre-eclampsia impair the maternal immune systems’ ability to correctly regulate the depth of EVT invasion.
Moreover, we identified enriched ligand interactions with ITGβ1 as important in regions of increta22. This is in keeping with the deep EVT invasion seen in PAS, as ITGβ1 promotes EVT invasion by activating EMT78,79. On the other hand, several studies have shown ITGβ1 is downregulated in the placenta of patients with pre-eclampsia, which contributes to reduced invasive capabilities of EVTs80,81,82. Furthermore, both ITGβ1 and CD44 are promoters of EVT invasion and were upregulated in increta regions22, both of which are decreased in pre-eclampsia83.
ECM degradation is an essential step in normal placentation in order to allow placental villi to invade the decidua and myometrium, which is facilitated by MMP such as MMP-9 and MMP-284. These MMP-s are expressed in EVTs and contribute to their invasive capabilities85. In PAS placentas, these MMP-s are increased86,87, suggesting increased ECM degradation in these cases is an important mechanism for increased cell migration. This was supported by our finding of increased ECM degradation in increta regions22. On the other hand, placentas of women with pre-eclampsia have absent or weak staining for MMP-988,89,90. These findings suggest PAS is characterised by increased ECM degradation which removes the physical barriers for EVT invasion into the myometrium, likely as a result of prior uterine injury, while reduced MMPs in pre-eclampsia EVTs mean they are unable to break the barriers of the myometrium effectively, resulting in shallow invasion.
Furthermore, pregnancy associated plasma protein A (PAPPA), another metalloproteinase which is a marker of deep trophoblast invasion64 was overexpressed in PAS regions in our study22, and others91,92. PAPPA cleaves insulin like growth factor 1 (IGF-1), which was also increased in increta regions22, promoting cell growth and EVT invasion93. Conversely, both PAPPA and IGF-1 are decreased in placentas of pregnancies complicated by pre-eclampsia94,95,96. This suggests dysregulation of the PAPPA-IGF pathway, which is important for normal EVT invasion, contributes to the abnormal depth of EVT invasion seen in PAS and pre-eclampsia.
Key transcription factors in PAS and pre-eclampsia
We inferred transcription factor activities of differentially expressed genes in increta regions and found dysregulated signalling of several of these may contribute to deep EVT invasion22. To our knowledge, these have not been previously been explored in PAS. Many of these transcription factors, such as AP-1 and its subunits ATF3 and JUNB97 as well as EGR1, CEPBP, SIRT1 and SP198,99,100,101, show the opposite expression pattern to previous studies in pre-eclampsia.
AP-1 and its subunits (ATF3, JUNB) appear to play an important role in increased EVT invasion in PAS, as we demonstrated increased expression of these transcription factors22. In contrast, in pre-eclampsia AP-1 subunits are downregulated102. Specifically, we showed AFT3, which is highly expressed in EVTs64, is significantly increased in PAS regions22, while others have shown ATF3 expression is reduced in pre-eclampsia97. In pre-eclampsia, decreased ATF-3 may result from chronic hypoxia, which leads to downstream events such as increased expression of anti-angiogenic factors and release of proinflammatory cytokines97. Therefore, it is possible that increased ATF3 in PAS contributes to the increased angiogenesis and vascularity seen in these cases in a reverse manor to that described in pre-eclampsia, as well as contributing to the local immune suppression we reported in increta regions22. This is supported by our finding that ATF-3 upregulates several genes involved in angiogenesis such as SERPINE 1 and 2 in increta regions103, as well as TIMP3 which conversely is downregulated in pre-eclampsia104.
Several other transcription factors showed the opposite expression pattern in pre-eclampsia and our PAS study. SP1, a transcription factor involved in many cellular processes, has been identified as a contributor to recurrent miscarriage with downregulation associated with increased trophoblast apoptosis105. SP1 overexpression results in accelerated trophoblast cell proliferation and invasion106. In keeping with this, we found SP1 was upregulated in PAS regions22. In pre-eclampsia decidual SP1 is downregulated, which results in abnormal decidualisation98. Interestingly, SP1 downregulation results in reduced IGF-1 which, as described above, is reduced in pre-eclampsia and increased in PAS98.
CEBPB, a negative regulator of apoptosis, was also upregulated in PAS compared to control regions22. Conversely, CEBPB is significantly reduced in the EVTs in pre-eclampsia100. Hence its reduced expression in pre-eclampsia and increase in PAS is in keeping with our finding of an anti-apoptotic environment in PAS and increased trophoblast apoptosis in pre-eclampsia107. Finally, SIRT1 a transcription factor involved in protection of vascular endothelial cells from oxidative stress, inflammation and senescence108, was significantly decreased in increta regions compared to controls22. SIRT1 knockdown results in enhanced invasion and migration of trophoblast cells, suggesting SIRT1 is a negative regulator of angiogenesis and EVT invasion109. In contrast, placental SIRT1 expression is increased in severe pre-eclampsia compared to both mild pre-eclampsia and healthy placentas101, further supporting the role of SIRT1 in trophoblast regulation.
These findings suggest that many of the transcription factors we found to be upregulated in PAS show mirror findings to the findings reported in pre-eclampsia. This further supports that these transcription factors may contribute to increased cell migration, proliferation and EVT invasion beyond the superficial myometrium in PAS.
Discussion
Throughout this manuscript we have highlighted an inverse correlation between many of the biological processes altered in PAS and in pre-eclampsia (Fig. 2). This suggests that the majority of alterations associated with abnormal placental invasion, such as immune dysfunction, increased cell proliferation and ECM degradation, show the opposite expression pattern in pre-eclampsia (Fig. 2). These biological comparisons of two important obstetric conditions provide further insights into the dysregulated processes that support EVT invasion in PAS as a result of myometrial scarring.
Pre-eclampsia and PAS are both conditions where abnormal decidualisation results in defective EVT invasion9. The cause of these decidual defects leading to abnormal placental invasion differ significantly between PAS and pre-eclampsia (Fig. 1). In pre-eclampsia, shallow trophoblast invasion is thought to result from decidual resistance, defined as the inability of the decidua to undergo the normal adaptations required to facilitate physiological placental invasion110, while in PAS, defective decidualisation occurs secondary to local uterine injury which facilitates pathological invasion15. This is further supported by epidemiological data suggesting that PAS may be protective against the development of gestational hypertension or pre-eclampsia111,112.
In pre-eclampsia, decidual resistance leads to shallow EVT invasion, impaired spiral artery remodelling and subsequent placental hypoxia and ischaemia resulting in the release of cytokines and anti-angiogenic factors from the syncytiotrophoblast, leading to maternal and foetal complications110. The importance of the decidua in this process has been demonstrated by studies showing that the transcriptional profile of EVT’s cultured from women with pre-eclampsia revert to normal migration and invasion when removed from the decidua113,114.
PAS is considered a secondary uterine disorder as a result of iatrogenic injury which leads to absent or defective decidua and therefore is predominantly seen in women with a prior Caesarean delivery and placenta previa15,115. The molecular changes seen at the maternal-foetal interface in PAS are likely secondary responses to myometrial injury21,22,116 and in some cases uterine scar dehiscence16,17,18. As gestation advances and the myometrium thins in an area of scar dehiscence, this facilitates access of EVTs to the outer myometrium (Fig. 1)9,16,17. EVTs then remodel maternal spiral arteries close to the uterine serosa and bladder interface117,118,119 as demonstrated by the upregulation of genes involved in angiogenesis and spiral artery remodelling, such as EGFL6, APOLD1, EDNRB, and CD36 in PAS21,22. This deep remodelling of spiral arteries and vesicouterine neovascularisation corresponds to the abnormalities of the utero-placental circulation seen on ultrasound and contributes to the surgical complexity of PAS cases15.
Future directions
One of the major limitations of translational and multi-omic based PAS studies is the use of inappropriate controls, such as healthy gestational aged matched controls and pre-term birth38,39,120, which limits meaningful interpretations of the results. To overcome this limitation, we and others have used internal controls from the same case to compare accreta and increta regions or adherent and non-adherent areas22,28. As a field it is imperative that we all agree on appropriate controls going forwards which could also include myometrium from cases of scar dehiscence with no evidence of invasion.
Alternatively, a comparative biology approach, as presented here may yield important information regarding the underlying pathophysiology of both PAS and pre-eclampsia using a multi-omics systems biology approach. This approach should focus on early pregnancy specimens in PAS, as to our knowledge nearly all studies of PAS at present are restricted to late pregnancy. An exploration of decidual, immunological and trophoblast changes in early pregnancy is required to improve understanding of the molecular pathogenesis of PAS. While this presents experimental challenges, Caesarean scar pregnancies—which have a very high risk of proceeding to PAS—are the best early pregnancy correlate121. Such an approach would allow us to understand the evolution of PAS as pregnancy progresses.
Particular focus on the immune microenvironment at the feto-maternal interface and how this changes over time during a PAS pregnancy will be important. In normal pregnancy, decidual immune cells undergo a dynamic shift from early pregnancy to late, with natural killer cells and T-cells promoting a shift from a lymphoid dominant to myeloid dominant decidua from 12 to 24 weeks122. However how or if this is altered in PAS is currently not understood. While we and others have identified difference in expression of common immune checkpoints in PAS such as PD-1 and CTLA-422, the biological significance and role of other immune checkpoints such as TIGIT, LAG3 and TIM4 in PAS warrants further exploration. TIGIT, which has been studied extensively in oncology and is expressed by T-cells at the maternal-foetal interface, definitely merits further exploration123,124.
Comparative biology as presented here has several strengths and limitations. By using the extensive literature available for a more prevalent obstetric condition, we were able to better contextualise the intricate cellular interactions observed in PAS, a rare disease. Demonstrating the inverse biological processes in these two important obstetric conditions, which are associated with significant maternal morbidity and mortality, further supports our proposed biological explanations for the abnormal placental adherence and invasion seen in PAS. However, this approach is also associated with limitations. While we have shown many contrasts between PAS and pre-eclampsia, comparing current PAS work to the pre-eclampsia literature is a simplified method that does not account for heterogeneity and methodological variances between studies. Therefore, while many contrasts between PAS and pre-eclampsia are highly plausible based on the work presented here, validation of these findings are needed. Nonetheless, we have shown a biological comparison framework is valuable for understanding rare disease, generating hypothesis and identifying further areas for research. We suggest future studies compare, at the single-cell level and using spatial technology, PAS and pre-eclampsia to validate the findings presented here. In particular, the decidual abnormalities that either permit abnormal or resist normal placental villi invasion in these two conditions warrant exploration.
In conclusion, many of the underlying biological processes we found were dysregulated in PAS are mirrored in pre-eclampsia. This comparative biology approach has allowed us to gain further understanding of the regulators of placental invasion. Both conditions are associated with significant maternal morbidity and mortality, hence understanding their pathophysiology is of clinical importance. Furthermore, the genes and proteins identified in our study as contributing to the pathophysiology of PAS, such as AP-1 signalling, fibronectin and CD44, may warrant further investigation in pre-eclampsia.
References
Liu, Y. et al. Single-cell RNA-seq reveals the diversity of trophoblast subtypes and patterns of differentiation in the human placenta. Cell Res. 28, 819–832 (2018).
Burton, G. J. & Fowden, A. L. The placenta: a multifaceted, transient organ. Philos. Trans. R. Soc. Lond B Biol. Sci. 370, 20140066 (2015).
Shi, Q. J., Lei, Z. M., Rao, C. V. & Lin, J. Novel role of human chorionic gonadotropin in differentiation of human cytotrophoblasts. Endocrinology 132, 1387–1395 (1993).
Yabe, S. et al. Comparison of syncytiotrophoblast generated from human embryonic stem cells and from term placentas. Proc. Natl Acad. Sci. USA 113, E2598–E2607 (2016).
Burton, G. J., Cindrova-Davies, T., Yung, H. W. & Jauniaux, E. Hypoxia and reproductive health: oxygen and development of the human placenta. Reproduction 161, F53–f65 (2021).
Moser, G. & Huppertz, B. Implantation and extravillous trophoblast invasion: from rare archival specimens to modern biobanking. Placenta 56, 19–26 (2017).
Pijnenborg, R., Vercruysse, L. & Hanssens, M. The uterine spiral arteries in human pregnancy: facts and controversies. Placenta 27, 939–958 (2006).
Ma, Y. et al. Placental endovascular extravillous trophoblasts (enEVTs) educate maternal T-cell differentiation along the maternal-placental circulation. Cell Prolif. 53, e12802 (2020).
Jauniaux, E., Jurkovic, D., Hussein, A. M. & Burton, G. J. New insights into the etiopathology of placenta accreta spectrum. Am. J. Obstet. Gynecol. 227, 384–391 (2022).
Sokratous, N. et al. Screening for pre-eclampsia by maternal serum glycosylated fibronectin at 11-13 weeks’ gestation. Ultrasound Obstetrics Gynecol. 62, 504–511 (2023).
Higgins, M. F., Monteith, C., Foley, M. & O’Herlihy, C. Real increasing incidence of hysterectomy for placenta accreta following previous caesarean section. Eur. J. Obstetrics Gynecol Reprod. Biol. 171, 54–56 (2013).
Jauniaux, E., Chantraine, F., Silver, R. M. & Langhoff-Roos, J. FIGO consensus guidelines on placenta accreta spectrum disorders: Epidemiology. Int. J. Gynaecol. Obstetrics 140, 265–273 (2018).
Roeder, H. A., Cramer, S. F. & Leppert, P. C. A look at uterine wound healing through a histopathological study of uterine scars. Reprod. Sci. 19, 463–473 (2012).
Jauniaux, E., Grønbeck, L., Bunce, C., Langhoff-Roos, J. & Collins, S. L. Epidemiology of placenta previa accreta: a systematic review and meta-analysis. BMJ Open 9, e031193 (2019).
Jauniaux, E., Hussein, A. M., Einerson, B. D. & Silver, R. M. Debunking 20(th) century myths and legends about the diagnosis of placenta accreta spectrum. Ultrasound Obstetrics Gynecol. 59, 417–423 (2022).
Jauniaux, E. et al. Searching for placenta percreta: a prospective cohort and systematic review of case reports. Am. J. Obstet. Gynecol. 226, 837.e831–837.e813 (2022).
Einerson, B. D. et al. Ultrasonography of the explanted uterus in placenta accreta spectrum: correlation with intraoperative findings and gross pathology. Obstet. Gynecol. 141, 544–554 (2023).
Einerson, B. D. et al. Placenta accreta spectrum disorder: uterine dehiscence, not placental invasion. Obstet. Gynecol. 135, 1104–1111 (2020).
Adu-Bredu, T. K. et al. Three-dimensional volume rendering ultrasound for assessing placenta accreta spectrum severity and discriminating it from simple scar dehiscence. Am. J. Obstet. Gynecol. MFM 6, 101321 (2024).
Hecht, J. L. et al. Classification and reporting guidelines for the pathology diagnosis of placenta accreta spectrum (PAS) disorders: recommendations from an expert panel. Mod. Pathol. 33, 2382–2396 (2020).
Afshar, Y. et al. Placenta accreta spectrum disorder at single-cell resolution: a loss of boundary limits in the decidua and endothelium. Am. J. Obstet. Gynecol. 230, 443.e1–18 (2024).
Bartels, H. C. et al. Spatial proteomics and transcriptomics of placenta accreta spectrum. bioRxiv, 2024.2003.2021.585167 (2024).
Jauniaux, E., Ayres-de-Campos, D., Langhoff-Roos, J., Fox, K. A. & Collins, S. FIGO classification for the clinical diagnosis of placenta accreta spectrum disorders. Int. J. Gynaecol. Obstet. 146, 20–24 (2019).
Shamshirsaz, A. A. et al. Maternal morbidity in patients with morbidly adherent placenta treated with and without a standardized multidisciplinary approach. Am. J. Obstet. Gynecol. 212, 218.e211–219 (2015).
Bartels, H. C. et al. Association of implementing a multidisciplinary team approach in the management of morbidly adherent placenta with maternal morbidity and mortality. Obstet. Gynecol. 132, 1167–1176 (2018).
Hypertension in pregnancy: diagnosis and management. Guideline available at https://www.nice.org.uk/guidance/ng133. Accessed March 2024.
Rolnik, D. L. et al. Aspirin versus placebo in pregnancies at high risk for preterm preeclampsia. N Engl J Med. 377, 613–622 (2017).
Ashraf, U. M., Hall, D. L., Rawls, A. Z. & Alexander, B. T. Epigenetic processes during preeclampsia and effects on fetal development and chronic health. Clin. Sci. 135, 2307–2327 (2021).
Tan, M. Y. et al. Comparison of diagnostic accuracy of early screening for pre-eclampsia by NICE guidelines and a method combining maternal factors and biomarkers: results of SPREE. Ultrasound Obstet. Gynecol. 51, 743–750 (2018).
Huppertz, B. Placental origins of preeclampsia: challenging the current hypothesis. Hypertension 51, 970–975 (2008).
Natenzon, A., McFadden, P., DaSilva-Arnold, S. C., Zamudio, S. & Illsley, N. P. Diminished trophoblast differentiation in early onset preeclampsia. Placenta 120, 25–31 (2022).
Liu, H., Wang, W. & Liu, C. Increased expression of IFN-γ in preeclampsia impairs human trophoblast invasion via a SOCS1/JAK/STAT1 feedback loop. Exp. Ther. Med. 21, 112 (2021).
Goldman-Wohl, D. & Yagel, S. Regulation of trophoblast invasion: from normal implantation to pre-eclampsia. Mol. Cell Endocrinol. 187, 233–238 (2002).
Hubel, C. A. Oxidative stress in the pathogenesis of preeclampsia. Proc. Soc. Exp. Biol. Med. 222, 222–235 (1999).
Myatt, L. & Cui, X. Oxidative stress in the placenta. Histochem. Cell Biol. 122, 369–382 (2004).
Levine, R. J. et al. Circulating angiogenic factors and the risk of preeclampsia. N. Engl. J. Med. 350, 672–683 (2004).
Vaisbuch, E. et al. Circulating angiogenic and antiangiogenic factors in women with eclampsia. Am. J. Obstet. Gynecol. 204, 152.e151–159 (2011).
McNally, L. et al. Up-regulated cytotrophoblast DOCK4 contributes to over-invasion in placenta accreta spectrum. Proc. Natl Acad. Sci. USA 117, 15852–15861 (2020).
Hecht, J. L., Karumanchi, S. A. & Shainker, S. A. Immune cell infiltrate at the utero-placental interface is altered in placenta accreta spectrum disorders. Arch. Gynecol. Obstet. 301, 499–507 (2020).
Murrieta-Coxca, J. M. et al. Identification of altered miRNAs and their targets in placenta accreta. Front. Endocrinol. 14, 1021640 (2023).
Liu, J., Lv, S. S., Fu, Z. Y. & Hou, L. L. Baicalein enhances migration and invasion of extravillous trophoblasts via activation of the NF-κB pathway. Med. Sci. Monit. 24, 2983–2991 (2018).
Vaughan, J. E. & Walsh, S. W. Activation of NF-κB in placentas of women with preeclampsia. Hypertens. Pregnancy 31, 243–251 (2012).
Huber, A. V., Saleh, L., Bauer, S., Husslein, P. & Knöfler, M. TNFalpha-mediated induction of PAI-1 restricts invasion of HTR-8/SVneo trophoblast cells. Placenta 27, 127–136 (2006).
Ye, Y. et al. Role of plasminogen activator inhibitor type 1 in pathologies of female reproductive diseases. Int. J. Mol. Sci. 18, 1651 (2017).
Kent, L. N., Rumi, M. A., Kubota, K., Lee, D. S. & Soares, M. J. FOSL1 is integral to establishing the maternal-fetal interface. Mol. Cell Biol. 31, 4801–4813 (2011).
Kang, J. et al. The emerging role of EGFL6 in angiogenesis and tumor progression. Int. J. Med. Sci. 17, 1320–1326 (2020).
Admati, I. et al. Two distinct molecular faces of preeclampsia revealed by single-cell transcriptomics. Med 4, 687–709.e687 (2023).
Kauma, S. W., Bae-Jump, V. & Walsh, S. W. Hepatocyte growth factor stimulates trophoblast invasion: a potential mechanism for abnormal placentation in preeclampsia. J. Clin. Endocrinol. Metab. 84, 4092–4096 (1999).
Fan, Z. et al. The vascular gene Apold1 is dispensable for normal development but controls angiogenesis under pathological conditions. Angiogenesis 26, 385–407 (2023).
Junus, K. et al. Gene expression profiling of placentae from women with early- and late-onset pre-eclampsia: down-regulation of the angiogenesis-related genes ACVRL1 and EGFL7 in early-onset disease. Mol. Hum. Reprod. 18, 146–155 (2012).
Tong, J. et al. Transcriptomic profiling in human decidua of severe preeclampsia detected by RNA sequencing. J. Cell Biochem. 119, 607–615 (2018).
Wei, L., Liping, Z. & Suya, K. Expression of insulin-like growth factor binding protein-3 in HELLP syndrome. BMC Pregnancy Childbirth 23, 778 (2023).
Kristensen, K., Larsson, I. & Hansson, S. R. Increased cystatin C expression in the pre-eclamptic placenta. Mol. Hum. Reprod. 13, 189–195 (2007).
Trowsdale, J. & Betz, A. G. Mother’s little helpers: mechanisms of maternal-fetal tolerance. Nat. Immunol. 7, 241–246 (2006).
Dunk, C. E. et al. Human leukocyte antigen HLA-C, HLA-G, HLA-F, and HLA-E placental profiles are altered in early severe preeclampsia and preterm birth with chorioamnionitis. Am. J. Obstet. Gynecol. 227, 641.e641–641.e613 (2022).
Zhang, R. et al. PD-L1 enhances migration and invasion of trophoblasts by upregulating ARHGDIB via transcription factor PU.1. Cell Death Discov. 8, 395 (2022).
Veras, E., Kurman, R. J., Wang, T. L. & Shih, I. M. PD-L1 expression in human placentas and gestational trophoblastic diseases. Int. J. Gynecol. Pathol. 36, 146–153 (2017).
Schwede, S., Alfer, J. & von Rango, U. Differences in regulatory T-cell and dendritic cell pattern in decidual tissue of placenta accreta/increta cases. Placenta 35, 378–385 (2014).
Wedenoja, S. et al. Fetal HLA-G mediated immune tolerance and interferon response in preeclampsia. EBioMedicine 59, 102872 (2020).
Goldman-Wohl, D. S. et al. Lack of human leukocyte antigen-G expression in extravillous trophoblasts is associated with pre-eclampsia. Mol. Hum. Reprod. 6, 88–95 (2000).
Hiby, S. E. et al. Combinations of maternal KIR and fetal HLA-C genes influence the risk of preeclampsia and reproductive success. J. Exp. Med. 200, 957–965 (2004).
Zhang, Y. et al. The altered PD-1/PD-L1 pathway delivers the ‘one-two punch’ effects to promote the Treg/Th17 imbalance in pre-eclampsia. Cell Mol. Immunol. 15, 710–723 (2018).
Mittelberger, J. et al. The programmed cell death protein 1 (PD1) and the programmed cell death ligand 1 (PD-L1) are significantly downregulated on macrophages and Hofbauer cells in the placenta of preeclampsia patients. J. Reprod. Immunol. 157, 103949 (2023).
Arutyunyan, A. et al. Spatial multiomics map of trophoblast development in early pregnancy. Nature 616, 143–151 (2023).
Wang, R. et al. Decreased AGGF1 facilitates the progression of placenta accreta spectrum via mediating the P53 signaling pathway under the regulation of miR-1296-5p. Reprod. Biol. 23, 100735 (2023).
Axt-Fliedner, R. et al. The immunolocalization of Bcl-2 in human term placenta. Clin. Exp. Obstet. Gynecol. 28, 144–147 (2001).
Pathare-Ingawale, P. et al. Association between proNGF receptors and apoptotic factors in human placentae. Placenta 139, 43–48 (2023).
Kasture, V., Sundrani, D., Randhir, K., Wagh, G. & Joshi, S. Placental apoptotic markers are associated with placental morphometry. Placenta 115, 1–11 (2021).
Ishihara, N. et al. Increased apoptosis in the syncytiotrophoblast in human term placentas complicated by either preeclampsia or intrauterine growth retardation. Am. J. Obstet. Gynecol. 186, 158–166 (2002).
Genbacev, O., DiFederico, E., McMaster, M. & Fisher, S. J. Invasive cytotrophoblast apoptosis in pre-eclampsia. Hum. Reprod. 14(Suppl 2), 59–66 (1999).
Kadyrov, M., Kingdom, J. C. & Huppertz, B. Divergent trophoblast invasion and apoptosis in placental bed spiral arteries from pregnancies complicated by maternal anemia and early-onset preeclampsia/intrauterine growth restriction. Am. J. Obstet. Gynecol. 194, 557–563 (2006).
Wang, C. C. et al. Innate immune response by ficolin binding in apoptotic placenta is associated with the clinical syndrome of preeclampsia. Clin. Chem. 53, 42–52 (2007).
Buurma, A. et al. Preeclampsia is characterized by placental complement dysregulation. Hypertension 60, 1332–1337 (2012).
Blakey, H. et al. Pre-eclampsia is associated with complement pathway activation in the maternal and fetal circulation, and placental tissue. Pregnancy Hypertens. 32, 43–49 (2023).
Lokki, A. I. et al. Identification of complement factor H variants that predispose to pre-eclampsia: a genetic and functional study. BJOG Int. J. Obstet. Gynaecol. 130, 1473–1482 (2023).
Derzsy, Z., Prohászka, Z., Rigó, J. Jr., Füst, G. & Molvarec, A. Activation of the complement system in normal pregnancy and preeclampsia. Mol. Immunol. 47, 1500–1506 (2010).
Denny, K. J., Woodruff, T. M., Taylor, S. M. & Callaway, L. K. Complement in pregnancy: a delicate balance. Am. J. Reprod. Immunol. 69, 3–11 (2013).
Vento-Tormo, R. et al. Single-cell reconstruction of the early maternal-fetal interface in humans. Nature 563, 347–353 (2018).
Gundappa, M., Arumugam, V. A., Hsieh, H. L., Balasubramanian, B. & Shanmugam, V. Expression of tissue factor and TF-mediated integrin regulation in HTR-8/SVneo trophoblast cells. J. Reprod. Immunol. 150, 103473 (2022).
Shu, C. et al. Integrin β1 regulates proliferation, apoptosis, and migration of trophoblasts through activation of phosphoinositide 3 kinase/protein kinase B signaling. J. Obstet. Gynecol. Res. 47, 2406–2416 (2021).
Li, P. et al. microRNA-29b contributes to pre-eclampsia through its effects on apoptosis, invasion and angiogenesis of trophoblast cells. Clin. Sci. 124, 27–40 (2013).
Zou, A. X., Chen, B., Li, Q. X. & Liang, Y. C. MiR-134 inhibits infiltration of trophoblast cells in placenta of patients with preeclampsia by decreasing ITGB1 expression. Eur. Rev. Med. Pharmacol. Sci. 22, 2199–2206 (2018).
Wang, Q. et al. Single-cell transcriptional profiling reveals cellular and molecular divergence in human maternal-fetal interface. Sci. Rep. 12, 10892 (2022).
Su, M. T., Tsai, P. Y., Tsai, H. L., Chen, Y. C. & Kuo, P. L. miR-346 and miR-582-3p-regulated EG-VEGF expression and trophoblast invasion via matrix metalloproteinases 2 and 9. Biofactors 43, 210–219 (2017).
Isaka, K. et al. Expression and activity of matrix metalloproteinase 2 and 9 in human trophoblasts. Placenta 24, 53–64 (2003).
Soyama, H. et al. Placenta previa may acquire invasive nature by factors associated with epithelial-mesenchymal transition and matrix metalloproteinases. J. Obstet. Gynaecol. Res. 46, 2526–2533 (2020).
Wang, R. et al. Overexpressed LAMC2 promotes trophoblast over-invasion through the PI3K/Akt/MMP2/9 pathway in placenta accreta spectrum. J. Obstet. Gynaecol. Res. 49, 548–559 (2023).
Shokry, M., Omran, O. M., Hassan, H. I., Elsedfy, G. O. & Hussein, M. R. Expression of matrix metalloproteinases 2 and 9 in human trophoblasts of normal and preeclamptic placentas: preliminary findings. Exp. Mol. Pathol. 87, 219–225 (2009).
Amakye, D. et al. Extracellular matrix metalloproteinases inducer gene polymorphism and reduced serum matrix metalloprotease-2 activity in preeclampsia patients. Exp. Biol. Med. 248, 1550–1555 (2023).
Li, W., Mata, K. M., Mazzuca, M. Q. & Khalil, R. A. Altered matrix metalloproteinase-2 and -9 expression/activity links placental ischemia and anti-angiogenic sFlt-1 to uteroplacental and vascular remodeling and collagen deposition in hypertensive pregnancy. Biochem. Pharmacol. 89, 370–385 (2014).
Wang, F. et al. First trimester serum PAPP-A is associated with placenta accreta: a retrospective study. Arch. Gynecol. Obstet. 303, 645–652 (2021).
Desai, N. et al. Elevated first trimester PAPP–a is associated with increased risk of placenta accreta. Prenat. Diagn. 34, 159–162 (2014).
Zhong, Q. et al. Structural insights into the covalent regulation of PAPP-A activity by proMBP and STC2. Cell Discov. 8, 137 (2022).
Kalousová, M., Muravská, A. & Zima, T. Pregnancy-associated plasma protein A (PAPP-A) and preeclampsia. Adv. Clin. Chem. 63, 169–209 (2014).
Krantz, D. et al. Association of extreme first-trimester free human chorionic gonadotropin-beta, pregnancy-associated plasma protein A, and nuchal translucency with intrauterine growth restriction and other adverse pregnancy outcomes. Am. J. Obstet. Gynecol. 191, 1452–1458 (2004).
Lai, W. & Yu, L. Insulin-like growth factor 1 ameliorates pre-eclampsia by inhibiting zinc finger E-box binding homeobox 1 by up-regulation of microRNA-183. J. Cell Mol. Med. 27, 1179–1191 (2023).
Kaitu’u-Lino, T. J. et al. Activating Transcription Factor 3 is reduced in preeclamptic placentas and negatively regulates sFlt-1 (Soluble fms-Like Tyrosine Kinase 1), soluble endoglin, and proinflammatory Cytokines in Placenta. Hypertension 70, 1014–1024 (2017).
Zhang, Y. et al. Downregulation of decidual SP1 and P300 is associated with severe preeclampsia. J. Mol. Endocrinol. 60, 133–143 (2018).
Park, M. N. et al. The expression and activation of sex steroid receptors in the preeclamptic placenta. Int. J. Mol. Med. 41, 2943–2951 (2018).
Zhou, W. et al. Trophoblast cell subtypes and dysfunction in the placenta of individuals with preeclampsia revealed by single‑Cell RNA Sequencing. Mol. Cells 45, 317–328 (2022).
Zhang, H., Wei, X. & Li, M. The expression of Sirt1/FoxO1 pathway in the placenta of patients with preeclampsia and its connection with prognosis. J. Obstet. Gynaecol. 42, 3514–3521 (2022).
Zhou, W. et al. Characterizing immune variation and diagnostic indicators of preeclampsia by single-cell RNA sequencing and machine learning. Commun. Biol. 7, 32 (2024).
Chern, S. R. et al. Spatiotemporal expression of SERPINE2 in the human placenta and its role in extravillous trophoblast migration and invasion. Reprod. Biol. Endocrinol. 9, 106 (2011).
Ma, R., Gu, B., Gu, Y., Groome, L. J. & Wang, Y. Down-regulation of TIMP3 leads to increase in TACE expression and TNFα production by placental trophoblast cells. Am. J. Reprod. Immunol. 71, 427–433 (2014).
Tang, H. et al. Down-regulation of the Sp1 transcription factor by an increase of microRNA-4497 in human placenta is associated with early recurrent miscarriage. Reprod. Biol. Endocrinol. 19, 21 (2021).
Tian, F. et al. Effects of the SPI/lncRNA NEAT1 axis on functions of trophoblast and decidual cells in patients with recurrent miscarriage. Crit. Rev. Eukaryot. Gene Expr. 33, 47–60 (2023).
Calicchio, R. et al. Preeclamptic plasma induces transcription modifications involving the AP-1 transcriptional regulator JDP2 in endothelial cells. Am. J. Pathol. 183, 1993–2006 (2013).
Yang, Y. et al. Regulation of SIRT1 and its roles in inflammation. Front. Immunol. 13, 831168 (2022).
Lee, K. M., Seo, H. W., Kwon, M. S., Han, A. R. & Lee, S. K. SIRT1 negatively regulates invasive and angiogenic activities of the extravillous trophoblast. Am. J. Reprod. Immunol. 82, e13167 (2019).
Garrido-Gómez, T., Castillo-Marco, N., Cordero, T. & Simón, C. Decidualization resistance in the origin of preeclampsia. Am. J. Obstet. Gynecol. 226, S886–s894 (2022).
Li, L., Liu, L. & Xu, Y. Hypertension in pregnancy as a risk factor for placenta accreta spectrum: a systematic review incorporating a network meta-analysis. Arch. Gynecol. Obstet. 307, 1323–1329 (2023).
Wang, W. et al. Association between hypertensive disorders complicating pregnancy and risk of placenta accreta: a meta-analysis and systematic review. Hypertens. Pregnancy 37, 168–174 (2018).
Zhou, Y. et al. Reversal of gene dysregulation in cultured cytotrophoblasts reveals possible causes of preeclampsia. J. Clin. Investig. 123, 2862–2872 (2013).
Garrido-Gomez, T. et al. Defective decidualization during and after severe preeclampsia reveals a possible maternal contribution to the etiology. Proc. Natl Acad. Sci. USA 114, E8468–e8477 (2017).
Fitzpatrick, K. E. et al. Incidence and risk factors for placenta accreta/increta/percreta in the UK: a national case-control study. PloS ONE 7, e52893 (2012).
Zhou, J. et al. Uterine damage induces placenta accreta and immune imbalance at the maternal-fetal interface in the mouse. Placenta 119, 8–16 (2022).
Schwickert, A. et al. Maternal serum VEGF predicts abnormally invasive placenta better than NT-proBNP: a multicenter case-control study. Reprod. Sci. 28, 361–370 (2021).
Shainker, S. A. et al. Placenta accreta spectrum: biomarker discovery using plasma proteomics. Am. J. Obstet. Gynecol. 223, 433.e431–433.e414 (2020).
Tseng, J. J. et al. Differential expression of vascular endothelial growth factor, placenta growth factor and their receptors in placentae from pregnancies complicated by placenta accreta. Placenta 27, 70–78 (2006).
Laban, M., Ibrahim, E. A., Elsafty, M. S. & Hassanin, A. S. Placenta accreta is associated with decreased decidual natural killer (dNK) cells population: a comparative pilot study. Eur. J. Obstet. Gynecol. Reprod. Biol. 181, 284–288 (2014).
Kaelin Agten, A. et al. The clinical outcome of cesarean scar pregnancies implanted “on the scar” versus “in the niche. Am. J. Obstet. Gynecol. 216, 510.e511–510.e516 (2017).
Greenbaum, S. et al. A spatially resolved timeline of the human maternal-fetal interface. Nature 619, 595–605 (2023).
Wienke, J. et al. Human Tregs at the materno-fetal interface show site-specific adaptation reminiscent of tumor Tregs. JCI Insight 5, 18 (2020).
Yu, X. et al. The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat. Immunol. 10, 48–57 (2009).
Acknowledgements
Figure 1 and 2 created using Biorender (biorender.com).
Author information
Authors and Affiliations
Contributions
H.C.B., C.Y., P.D., J.Mc.C., V.Z., D.J.B. designed the study; H.C.B., C.Y., M.N., P.D., J.Mc.C., A.F., and D.J.B. performed research and literature review; H.C.B., S.H., P.D., K.M.C., A.F., V.Z., and D.J.B. analysed data; H.C.B., S.H., W.K., D.J.B. wrote the original paper and all authors reviewed and edited paper. HCB and DJB prepared Figs. 1, 2.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bartels, H.C., Hameed, S., Young, C. et al. Biological comparisons between pre-eclampsia and placenta accreta spectrum. npj Womens Health 2, 30 (2024). https://doi.org/10.1038/s44294-024-00034-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s44294-024-00034-y