Abstract
Here, we review how respiratory viruses function as molecular motors by exploiting their glycan receptor-binding and -destroying glycoproteins. The resulting virion motility drives their mucus penetration and exploration of cell surfaces for locations permitting induction of entry. We discuss the role of virus morphology, kinetic parameters of receptor engagement and receptor heterogeneity, summarize physical models for virion motility and identify key gaps in our understanding of these dynamic processes.
Similar content being viewed by others
Introduction
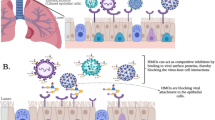
The typical infection route of respiratory viruses, using human influenza A virus (IAV) as an example, is shown in Fig. 1. Virus transmission mostly proceeds via the airborne route through inhalation of virus-loaded respiratory droplets and aerosols (1) or by direct contact. In the lumen of the upper or lower parts of the airways, virus particles land within seconds on a mucus layer covering the epithelial cells (2). Mucus is an established restriction factor for IAV infection in vivo and in vitro1,2. Virions in the mucus will be expelled by ciliary beating and need to traverse this layer rapidly. Their hemagglutinin (HA) binds to sialic acid (Sia) decoy receptors displayed on mucins which may delay this step (3) but the remarkable efficiency of aerosol transmission (e.g. a very low IAV dose of 0.7–3.5 PFU causes seroconversion in 50% of subjects3) suggests efficient escape from decoy receptors. There is growing evidence that epithelial cells are rapidly reached by directional virus motility resulting for IAV from the interplay between Sia-receptor binding by HA and cleavage by neuraminidase (NA) (4) as will be discussed in more detail below.
The numbered steps are described in the main text. Typical examples of glycan structures of the three major glycoconjugate groups are shown. Decoy receptors (3) are shown as an O-glycan-rich domain of soluble mucins but may also be present on the cell membrane, for instance on transmembrane mucins. Black arrows indicate virus motility. Clustering of glycosylated transmembrane proteins (7 and 8) may be crucial to signaling the induction of endocytic entry. Created in BioRender. Liu, M. (2025) https://BioRender.com/kxmkvsn.
While technical challenges have thus far prohibited determination of viral doses4 inhaled under natural conditions few viral particles are expected to bind per cell at the initial stages of infection. As a consequence, virions must be highly efficient in finding sites that permit endocytic entry, e.g. by clathrin-mediated endocytosis or macropinocytosis5. Notably, IAV particles do not rely on constitutive endocytosis at the initial site of binding (5) nor actively induce endocytosis at that site. Instead, as shown by live particle tracking6, binding leads to extensive cell surface roaming (6) followed in time by stalling of virus particles at as yet undefined “entry-competent” sites at which signaling (7) induces endocytosis (8) within a time-span of ~2 min6.
Clearly, mucus penetration and cell surface roaming are crucial steps to keep optimized in view of escaping not only mucus expulsion but also (adaptive) immunity by minimizing extracellular presence. In this review we discuss the intricate machinery that is conceptually shared by members of at least four virus families in order to facilitate virus particle motility. Their sialoglycan receptors are (abundantly) present on the extracellular face of virtually every cell membrane and the soluble proteins in the mucus layer that covers the epithelial cells. As a leading principle, based on ample evidence, we propose that sialoglycan-binding virus particles become permanently, but dynamically, receptor-associated shortly after entering the respiratory tract. Motility, for penetrating the mucus layer and subsequently on the cell surface to find entry-competent sites, is largely dependent on the kinetic parameters of the interaction of these viruses with their sialoglycan receptors and the abundance and distribution of these receptors. These interactions critically involve receptor binding as well as receptor destruction by enzymatic cleavage. These two activities are present in virus envelope-embedded glycoproteins but are organized in a constellation that differs between members of the four virus families. Still, as we will discuss, evolution of the balance between receptor binding and cleavage is a unifying principle in regulating virus motility.
As a side note, the virus families covered in this review also contain members that can enter Sia-independently. Paramyxoviruses either use Sia or protein receptors7,8. Probably all coronaviruses rely on protein receptors, while some in addition require glycan receptors for attachment and/or opening of the spike protein to allow protein receptor binding via the RBD9,10,11,12. Also some orthomyxoviruses enter cells Sia-independently via MHC-II13,14,15,16,17. Why viruses within a virus family have evolved to enter via different types of receptors is not known. While the sheer abundance of glycan receptors may seem attractive, this abundance also comes at a cost as detailed in this review. Within this review we only focus on those viruses within these families that contain both sialoglycan-binding and -cleavage activities.
Multivalent binding and receptor cleavage as drivers of virus motility
The first challenge for a virus to enter a cell is binding to an attachment receptor. Whereas many virus species attach to a specific protein receptor with high affinity18,19 (KD ranging from 0.025 to 25 nM), influenza viruses and several coronavirus and paramyxovirus species bind to terminally located Sias of glycan chains attached to proteins and lipids. To compensate for the low affinity of individual viral protein-glycan interactions20,21,22,23,24,25 (KD ranging from 0.02 to 50 mM), viruses form multiple simultaneous receptor interactions (referred to as multivalent binding) for establishing high avidity binding required for cell infection26,27,28,29. Virus binding is not confined to unique sialoglycans displaying the highest affinity but can be supported simultaneously by different receptors with a range of affinities, resulting in a heteromultivalent binding mode30. In this way, a virus can efficiently exploit structurally diverse sialoglycan repertoires that, while varying between cell types and host species, are heterogeneously distributed on cell surfaces. Importantly, high avidity virus binding by multiple low affinity interactions determines its highly dynamic interaction with the cell surface and distinguishes it from binding to a high-affinity protein receptor. It can be imagined as an entity clinging with many “weak hands” to a ceiling full of handles, that can move around by exchanging “grips”. This differs dramatically from the monovalent high-affinity interaction (tightly hanging to a “single grip”) of viruses binding to a protein receptor that will direct the co-endocytosis of virions, either directly at the binding site or after surfing in continuous tight association with a motile protein receptor to an endocytic site. On the other hand, sialoglycan-binding viruses can bind any protein and/or lipid decorated with the matching glycan receptors but need to find proper sites at which endocytic vesicle formation can take place by moving over a landscape of sialoglycan receptors31,32,33.
In addition to multivalent, low affinity binding to a sialoglycan-receptor surface, receptor-destroying activity has been shown to be essential for virus motility. Initially, receptor cleavage by viral envelope-embedded proteins was shown to prevent self-aggregation of virus particles and to promote shedding of newly formed virus particles by de-sialylation of viral envelopes and cell membranes34,35. Later, NA activity was shown to be important for the initiation of IAV infection in cells of differentiated mucus-secreting airway cell cultures36 and recently directional motility on receptor-coated surfaces was shown to be critically dependent on receptor-destroying enzymes37,38,39,40. Thus, two virus components (receptor binding protein, RBP; receptor destroy enzyme, RDE) and a diverse receptor repertoire form the fundamental building blocks for a molecular motor (Fig. 2a). Before turning to mechanistic details of virus motility we briefly summarize the considerable diversity that is observed in the organization and structure of the participating viral proteins as well as in their preferred sialoglycan receptors.
a The distribution of the indicated lectin, enzyme and fusion functions over different proteins is schematically depicted for each of the major virus groups that are discussed. HA, HEs and HEF share a common ancestry with an unknown “HEF-like” precursor (red letter) while the origin of other proteins (e.g. NA and HN) cannot be resolved on basis of primary sequence conservation. HEF, HA and isavirus HE116 form homotrimers, while corona- and torovirus HE are dimers45. Remnant domains that are still recognizable but have lost function are marked by dashed-line/red letter. Note that the esterase function has been lost in HA while keeping a weak lectin function at what is called a vestigial esterase site (VES). The preferred receptor usage is indicated116,117, but exceptions can occur. For instance, some murine CoVs display specificity for 4-O-acetylated Sia118. Created in BioRender. Liu, M. (2025) https://BioRender.com/kxmkvsn. b Relevant examples of structural modifications of Sias are shown. The Neu5Gc modification may abolish HA binding but does not occur in humans. O-acetylation also occurs on N- and O-glycans.
Diversity of molecular motor components
Nearly all mammalian cells are decorated by the sialic acid (Sia) receptors on which we focus here. They are a family of nine-carbon backbone monosaccharides that are mostly present as terminal residues of a wide diversity of cell surface-displayed glycans or glycans attached to secreted proteins41. Determining factors for specific binding to particular virus envelope glycoproteins are 1) specific enzymatic modifications of the Sia moiety28, 2) the type of glycosidic linkage with the sub-terminal residue and 3) the structure of the underlaying glycan42,43,44,45 (Figs. 1 and 2). Sialoglycans are the product of the consecutive activities of a large number of glycosyltransferases and other glycan-modifying enzymes that collectively synthesize a huge diversity of glycans constituting the cellular glycome46,47. The exact glycome composition is a major determinant of virus host species tropism and also determines the preferential site of infection within the respiratory or enteric tract. The three major glycan classes that can be distinguished are glycosphingolipids (GSLs), glycans linked to asparagine residues of proteins (N-glycans) and glycans linked to serine or threonine residues of proteins (O-glycans)(Fig. 1). A subset of O-glycans is densely spaced in, often repetitive, mucin domains of soluble and transmembrane mucins that form a decoy receptor layer protecting epithelial cells from infection1,2.
Envelope proteins from viruses from the families of Orthomyxoviridae and Paramyxoviridae and the order of Nidovirales have been demonstrated or suggested to display receptor destruction-driven motility on sialoglycan-coated surfaces20,37,38,39,40. Limited, but significant, primary sequence conservation and structural similarities suggest a common ancestry of Orthomyxoviridae envelope proteins (HA, hemagglutinin-esterase fusion protein [HEF] and hemagglutinin-esterase protein [HE]) and HE of some Nidovirales (Fig. 2a). Evolutionary trajectories remain questionable but have resulted in a remarkably diverse modular arrangement of receptor-binding and -destructing moieties in a single or in two proteins. Whereas a single enzymatic site for receptor cleavage is present, multiple glycan binding sites (lectin sites) are present in some cases. An unknown virus probably donated the protein ancestral to the HEF of influenza C and D (ICV, IDV)45. It possesses a lectin domain for binding to (7,)9-O-acetylated Sias and an esterase domain for cleaving the O-acetyl groups. HEF, or it’s precursor, is likely ancestral to HA of Influenza A and B viruses48 and the HE of infectious salmon anemia virus (ISAV), an orthomyxovirus species infecting fish49. ISAV HE has lost the fusion function of HEF which is replaced by a separate, non-related, fusion protein (F)50. Remarkably, binding and cleavage of ISAV HE is specific for 4-O-acetylated Sias50. In case of IAV/IBV, HEF has evolved more drastically, into a protein boasting a lectin site that interacts with α2-3 or α2-6 linked Sias of the N-acetylneuraminic acid type (Fig. 2b) that are not O-acetylated51. For receptor destruction, it has therefore acquired a separate NA protein that cleaves α2-3 or α2-6 linked Sias. The HEF esterase site was shown to be maintained in HA as a vestigial esterase site (VES) that is inactive but, despite marginal primary sequence conservation, still able to bind Sia at very low affinity52. The class I fusion proteins HEF and HA are organized into homo-trimers and expose a fusion peptide upon a low pH-induced conformational change. Some Toroviruses (e.g. BToV, PToV) and coronaviruses of the Embeco subgenus (e.g. BCoV, OC43, HKU1, PHEV), both belonging to the Nidovirales, followed a different evolutionary track in acquiring a potential motility machine. Also in these cases, a HE ancestrally related to HEF has been acquired, most likely by independent horizontal gene transfers45. However, the fusion function was lost and replaced by acquiring a separate fusion protein (spike). Notably, this homo-trimeric spike protein harbors, in contrast to ISAV F protein, a lectin domain for binding (7,)9-O-acetylated Sias and has evolved into the major receptor-binding protein while the esterase activity remains located in the HE protein with its quaternary structure changed into a dimer. As yet another variation, several paramyxoviruses use a set-up in which a single hemagglutinin-neuraminidase (HN) protein of unknown origin displays an active site that provides both binding and cleavage function44.
Adding to the complexity of molecular motors are the additional Sia-binding sites that can be present in all three virus groups. A subset of paramyxovirus HN53 and influenza virus NA54,55,56 proteins carry an additional lectin site named second sialic binding site (2SBS). Similarly, a subset of HE proteins (e.g. BCoV) have retained the lectin site from the enigmatic ancestor shared with HEF. The function of these secondary lectin sites was proposed to be mainly important for modulating receptor-destroying activity by directing glycans to the catalytic site55,57. Remarkably, loss of secondary lectin sites seems to be prominent for human strains. For all recorded IAV pandemics, the loss of a 2SBS occurred at, or shortly after, the start of a pandemic whereas a 2SBS is almost absolutely conserved in avian IAVs. Loss of a 2SBS has occasionally also been reported upon transfer of avian IAVs to other mammals58. Human coronavirus OC43, which likely derives from bovine coronavirus BCoV, was also associated with a loss of a functional lectin site in HE57. Finally, in contrast to avian paramyxovirus NDV, human paramyxovirus hPIV3 has lost its 2SBS59 although it is not clear whether this is a human host-specific adaptation.
Motility of spherical virus particles on receptor surfaces
Evolution of the arrangement and functionality of lectin sites and hydrolytically active sites in viral envelope proteins (Fig. 2) likely affects virus motility. The first experimental evidence for motility on the cell surface or in the mucus layer37,38,39 has been obtained for NA or HEF receptor-destruction-driven motility of, respectively, IAV and ICV particles. Theoretical models explaining directional motility of virus particles by an HA/NA “motor system” have been developed60,61,62,63. Motility of paramyxoviruses like Sendai virus (SeV) and Newcastle disease virus (NDV) was recently shown40 but a role for receptor binding and destruction in motility of coronaviruses20 has not yet been experimentally demonstrated. Different modes of (directional) motility are discussed below and depicted in a simplified way in Fig. 3.
a Random IAV motion over short distances occurs in absence of NA activity. b In presence of receptor-destroying activity, spherical or short cylindrical IAV or ICV particles show fast directional motility alternating with periods of random motion that may result in a shift of direction. c Filamentous IAV particles display crawling motion. d ICV filament rolls over their longitudinal axis (indicated by the thin bended arrows) and display change of direction by flapping (indicated by the thick bended arrow). Created in BioRender. Liu, M. (2025) https://BioRender.com/kxmkvsn.
Virus motility of IAV on the cell surface was first observed by Rust et al. who aimed to describe cell entry mechanisms exploited by IAVs by using live cell imaging6. Virus particles were shown to traverse the cell surface for minutes before stalling at a site at which entry only occurs after a lag phase of up to 2 min during which active signaling and recruitment of diverse endocytic machinery takes place. Sakai et al.37 subsequently also used live cell imaging to demonstrate IAV motility on artificial receptor-coated surfaces and on the surface of human erythrocytes. Importantly, directional motility was dependent on NA activity, and NA inhibitors were shown to reduce virus entry, strongly suggesting the need for virus motility to reach sites of endocytosis. Two types of motility were observed. In absence of NA activity particles moved slowly, staying confined to a small area and displaying frequent change of direction (random motion) (Fig. 3a). In presence of NA activity, random motion was interrupted by fast, largely directional, gliding motility by crawling (“stepping”) or rolling (Fig. 3b–d). Intermittent occurrence of the two types of motion allowed change of direction at the slow phase. Others used real-time kinetic analysis of IAV-receptor surface interactions by biolayer interferometry (BLI) revealing a crucial role for the balance between HA and NA in extensively clearing the receptor surface from sialoglycan receptors38. By the cooperative action of a small number of virus particles, imaginatively visualized as “lawn mowers” moving over the surface, receptor density becomes gradually decreased to levels at which virus particles dissociate. Recently, integrating confocal and atomic force microscopy, NA activity-dependent motility of IAV particles on the cell surface prior to cell entry64 was confirmed and refined.
High avidity multivalent binding (KD in the pM range33,65) results from monovalent HA-sialoglycan interactions with KD values as low as 1–50 mM66 (Table 1). These weak monovalent interactions have also been measured by atomic force microscopy67 and were shown by single-particle tracking to be, relative to multivalent interaction, abundant65,67. Expectedly, the low affinity of a monovalent interaction results in a binding equilibrium that is far to the unbound state at the low virus concentrations encountered in vivo. Conversion to bivalent binding will be rare but the resulting enhancement of binding avidity will accelerate formation of additional interactions and lead to virtually irreversible multivalent binding. In contrast to single-particle tracking, BLI is an “ensemble method” that mainly records the accumulation of multivalently associated particles. Receptor destruction by NA activity is almost completely restricted to particles that are first bound to the receptor surface by HA-Sia interactions38. Variation of the KD (equal to koff/kon) between IAV strains (1–50 mM) is determined by changes in their binding rate constant (kon) as the koff is highly conserved and results in a half-life of monovalent interactions of ~0.8 s66. This results in a dynamic binding mode where rapid exchange of Sias between HA and NA, results in progressive removal of Sia by NA activity that is essential for driving exploration of the cell surface in search for rare entry-competent sites. Motility on supported lipid bilayers was also microscopically observed for Sendai virus (SeV), the prototypical member of the respirovirus genus of the Paramyxoviridae68. Real-time kinetic analysis of receptor binding and clearance by paramyxoviruses suggested an IAV-like rolling mechanism for viruses that carry binding and cleavage functions of sialoglycan receptors in the single active site of the HN protein40. Up to date, reports on (cell surface) motility of coronaviruses have been lacking.
Motility of filamentous virus particles on receptor surfaces
The observations above for IAV were made on spherical or slightly cylindrical virus particles of ~100 nm diameter and 90–120 nm length (Table 1). However influenza viruses can display a filamentous morphology69, especially when directly isolated from hosts, while mostly spherical respiroviruses like SeV are pleiomorphic in displaying a diversity of diameters70. Interestingly, a mostly filamentous ICV, carrying a HEF protein that combines a receptor binding site and a separate receptor cleavage site in a single protein, was also shown to display motility on receptor-coated surfaces39. Rolling occurred over the longitudinal side and was unidirectional for a while after which “flapping”, by remaining attached to the receptor surface with the tip of the filament, can result in a change of direction (Fig. 3d). Similar to role of NA in IAV motility, the esterase activity of HEF was proposed to drive directional motility by clearing the virus trajectory from receptors and thereby preventing backward motion. In addition, the cylindrical shape of the particle will largely restrict sideward changes of direction that characterizes motility of spherical IAV particles. Filamentous ICV particles moved slower than spherical IAVs. Notably, this was not due to kinetic differences in binding and cleavage activities between ICV HEF and IAV HA and NA, nor by HEF combining both activities in one protein, while these are physically separated for HA and NA, but rather to differences in morphology: an ICV strain displaying spherical morphology showed fast directional motility, alternating with slow random motility, similar to spherical IAVs39.
As receptor binding and cleavage activity of ICV are organized in a single HEF protein, their distribution along the filament is identical. In contrast, HA and NA of a filamentous IAV particles have been shown to be asymmetrically distributed along the filament with NA being concentrated at one end. Analysis of filamentous IAV motility on receptor-coated coverslips revealed trajectories in a direction lateral to its long axis71 (Fig. 3c), in contrast to the orthogonal motility of filamentous ICV39 (Fig. 3d). Remarkably, motility of IAV filaments occurred strictly in a polarized orientation, moving away from the NA-rich pole at the back end of the filament. This crawling motion was NA activity-dependent. In agreement, staining the coverslips after the experiment with a lectin, specific for desialylated glycans, revealed receptor-depletion along the trail followed by the virus filament.
A recent study on SeV further emphasized the significance of virus morphology on motility72. In this case, as for ICV HEF, receptor binding and cleavage are organized in a single HN protein. SeV particles are spherical but display a diameter range of 100–400 nm. Single particle tracking showed small SeV particles (~129 nm diameter) displaying binding efficiency highly dependent on receptor density (GD1a embedded in supported lipid bilayer). Similar effects were previously observed when increasing receptor density using BLI38,73 or supported lipid bilayer assays74 for IAVs and paramyxoviruses40. Larger SeV particles (~165 nm diameter), however, were less dependent on receptor density and moved at a lower speed72. Presumably, an increased number of interactions at the extended contact area of a larger particle increases binding efficiency while decreasing motility.
Motility of virus particles through mucus layers
Receptor-destroying activity is also an essential driver of directional mucus penetration by both filamentous and spherical virus particles71,75,76. The mucus layer that covers respiratory epithelia becomes gradually thinner from nasal cavity to bronchioles and is absent from alveoli. Soluble mucins like muc5AC and muc5B multimerize head-to-tail into filaments forming a mesh-like gel layer with a pore size of ~100–500 nm1,2. The extended filaments are densely covered with sialylated O-glycans, interspersed with N-glycans, and may form linear tracks to which viruses can bind and move along in dependence of receptor-destroying activity2. Despite the lack of detailed glycomic analyses, mucus is generally assumed to display abundant α2-3-linked Sias (2-3Sia)77. Therefore, mucus has been proposed to particularly block penetration by avian IAVs that preferably bind 2-3Sia43. Human IAVs prefer binding to α2-6-linked Sias (2-6Sia)43 and may thus be less affected by the thick mucus layer of the upper respiratory tract. However, this has poorly been investigated in detail and raises questions. For instance, human paramyxoviruses specifically bind to α2-3 linked Sias but can efficiently infect the upper respiratory tract78 indicating they can efficiently deal with a thick mucus layer that is rich in α2-3 linked Sias and which might suggest that avian IAVs should be capable of this too. Sialylated tracks provided by linear mucin filaments seem, more than the Sia landscape on the cell surface, ideally suited for guiding directional rolling motility of spherical particles. Still, the filaments form a network layer and high-magnification live-imaging only showed that filamentous IAVs display directional motility in a mucus layer without detailing the precise interaction with mucin filaments71. Overall it can be concluded that mucus penetration is directional and requires receptor-destroying activity but its mechanistic similarity to cell surface motility needs further exploration.
Theoretical models for virus motility
Physical models aiming to capture the observed virus motility phenomena have recently been developed. Sakai et al.37 argued that the energy for the observed random motion of spherical IAV particles on a grid-like receptor surface originates from the impact of water molecules. Akin to the Brownian motion of small particles in solution, Brownian ratchet models79,80 could apply to such virus motility. Directional motility requires an external energy source which in this case is provided by receptor-cleavage activity. Possibly, virus particles move, on average, away from receptor-depleted areas towards unvisited areas to “leave behind the burnt-bridge” (sometimes plastically described as a “lawnmower” going over a receptor surface). While burnt-bridge Brownian ratchet theories are frequently used to describe molecular motors, other physical models have recently been developed for influenza virus motility. Ziebert et al. have developed more elaborate models for IAV61 (spherical or slightly cylindrical virions and carrying binding and cleavage sites in separate proteins) and ICV60 (cylindrical or filamentous virions with binding and cleavage sites in the same protein). Differences in polarization of linkers (i.e. receptor-glycoprotein interactions) at the contact zone at the front and rear end of the particle give rise to a torque that drives rolling in the front-end direction. On basis of this model they proposed that rolling, orthogonal to the rotational axis of the particle, is inevitable in the presence of viral glycoproteins that bind and enzymatically cleave their ligands on the receptor surface. In their models, parameters were introduced to investigate the effects of variations in receptor density, binding strength, cleavage activity and virion morphology (cylindrical vs spherical). Directionality and reversal of motion appears to be affected by all these parameters.
For motility of filamentous IAVs through mucus layers, lateral to its long axis, a different model was proposed. Cases were investigated for either a polarized or uniform distribution of HA and NA62. For both cases, a minimal filament length was predicted to be required to switch from diffusional to directional motion. Polarized organization of NA at the rear-end resulted in more robust directionality than a uniform distribution. Also, too low or too high HA-receptor affinity both resulted in extreme loss of motility but, remarkably, locomotion was predicted to be largely insensitive to changes in NA activity. Finally, Ziebert et al. have proposed a model for mucus penetration by filamentous viruses63 that involves an alternation between a symmetrical rod-like shape and curved shapes (described as “toro-elastic”). These morphological changes are predicted to be induced by short-range interactions between neighboring spikes. Rotation of such curved states will be invoked by receptor cleavage and lead to a propulsion of changes in filament shape that promotes virus motility. Collectively, these predictions impose another level of complexity on the evolutionary fine-tuning of the HA/NA balance that is supposed to be a crucial factor in virus fitness and a barrier for cross-species transmission32.
Physical models are built on measured or predicted kinetic and physical parameters and can be experimentally challenged for further improvement. Yet, number and range of variables affecting in vivo virus motility is expectedly huge and often reluctant to quantification or experimental verification. For instance, mucus layers are difficult to reconstruct in vitro and virus morphology cannot easily be changed while remaining largely unexplored for its role in infection. A solely spherical IAV morphology has been suggested to be a cell culture adaptation but solid proof for this is lacking. Notably, spherical particles have been shown to efficiently penetrate a mucus layer in strict dependence on NA activity in apparent contrast to one of the model predictions mentioned above62.
Towards improved models of virus binding dynamics and motility
We next discuss qualitative and quantitative data on viral and cellular factors affecting virus motility. Multivalent virus-binding avidity, usually determined in absence of receptor destroying activity, appears virtually irreversible with dissociation constants (KD) as low as a few pM65,81. These avidities, however, often correspond poorly to infection efficiency82,83,84,85,86. Apparently, infection efficiency (also) depends on steps beyond initial binding, such as virus motility and cell surface roaming, or on the other hand virus stalling and subsequent induction of endocytosis (Fig. 1, steps 7–9). All these steps are determined by the concerted action of receptor binding and destruction, which coevolve to maintain a supposed optimal balance between them and their targeted receptor repertoire66,81,87,88. This balance is primarily determined by the kinetic binding and cleavage parameters of the individual proteins that also form the basis for the theoretical models. Unfortunately, only very little information on these parameters is available for a limited set of soluble glycans. This poses a challenge as the cell surface displays a diverse mixture of glycans of which many will contribute by an imperative heteromultivalent binding mode simply because glycans displaying higher and lower affinity can be located in close proximity. It seems unlikely that receptor binding and destruction can be optimally balanced for every individual receptor. Actually, the observed particle stalling on the cell surface may occur at places (microdomains) with a receptor composition (type and density) that is unbalanced and favors prolonged binding.
Table 1 presents a concise overview of, currently still limited, knowledge on virus parameters that may affect binding and motility of which some are discussed below. Recently, IAV HA-Sia monovalent receptor binding affinities were shown to fall within a relatively large 1–50 mM range despite their ability to support equally efficient infection levels66. In contrast, for coronavirus OC43 spike protein a much higher affinity (~50 µM) has been reported20. Remarkably, the dissociation rate constant (koff) for OC43, HPIV1, HPIV2, HPIV3 and for a range of IAVs were all very similar, resulting in a half-life time of ~0.6–3 s. As a consequence, variation of the association rate is the main variable in determining affinity (KD = koff/kon). Possibly, the large difference between OC43 and IAVs reflects differences in density of available receptors, with OC43 being dependent on binding to possibly less abundant O-acetylated Sias. Also, the number of interactions involved in multivalent binding may vary due to differences in density and motility of the receptor binding proteins in the viral envelope. Whereas HA and NA of IAV are tightly packed on the viral envelope, just as what is observed for paramyxoviruses89, this appears less well established for coronaviruses, which, based on electron microscopy, seem to display more variable densities of spike proteins90.
In many cases, modeling of binding dynamics still suffers from the current lack of precisely determined kinetic parameters. For example, kinetic parameters of secondary lectin sites of low affinity, for instance on NA of IAV (2SBS), HA of IAV (VES) and HE of BCoV (lectin site) should be considered in modeling of virus motility but have either not yet been determined or only to a limited extent (Table 1) without a systematic comparison of different strains. In addition, HEF of ICV/IDV and HN of NDV (2SBS) represent situations where a major lectin site and active site are present in the same protein. For HN, the active site also functions as the major site for attachment and a similar situation was observed for some IAV strains in which NA active site was mainly responsible for91, or contributing to38,92, binding.
Another important factor to consider is the accessibility and potential clustering of receptor binding and destroying enzymes. Whereas reported kinetic parameters are determined by the use of either soluble glycans or soluble proteins, natural multivalent binding takes place between receptor-coated surfaces and membrane-embedded viral proteins. This differs dramatically from a situation in which one of the components is accessible by diffusion in solution. When both are surface bound, density and a more restricted orientation will determine accessibility of viral proteins to sialoglycan receptors. Importantly, accessibility may differ between receptor-binding and receptor-destroying proteins and have a large impact on their balance towards specific receptors. NA of IAV often appears to be organized into a few clusters93,94 which has not been observed for coronavirus HE. HE of coronavirus has a ~2-fold lower height than its spike (Table 1) which will decrease accessibility, and thereby cleavage activity, for short glycans. HA and NA are commonly of similar height but deletions in the NA stalk region, making it shorter, seem to be an adaption of IAV strains originating from waterfowl to poultry. The resulting reduction in accessibility of NA to glycans might be a specific way of altering the HA/NA balance73.
Finally, but of utmost importance, the lipid biolayers that form the viral envelope and cell membrane are highly fluid. Membrane-embedded proteins and lipids can display a high rate of lateral diffusion. Lipids can migrate at a rate as high as 1 µm per second, depending on the local lipid composition of the membrane, and transmembrane proteins can also diffuse at high rates but motility can be restricted by anchoring of cytoplasmic domains to the cytoskeleton or protein multimerization. However, depending on lipid composition, lipids are also found to be organized into lipid micro- and nano-domains where motility is restricted. Such domains, and their selective protein content have been shown to play an important role in IAV and SeV binding avidity, motility and signaling of endocytosis72,95,96. Diffusion of receptors will obviously affect their local density and thereby the kinetics of multivalent binding. It will, for instance, complicate the modeling of an “effective molarity” which was defined as the volume that an unbound ligand in a partly bound complex can probe29,97. One consequence of a fluid membrane for heteromultivalent binding has been shown in lectin binding studies. Lectins usually display two or more glycan binding sites in a single protein or a stable lectin multimer. Like recently shown for IAV, also lectins can bind to different receptors with different affinities. It was shown that initial multivalent binding occurs to receptors of higher affinity but in time these can be exchanged with receptors of lower affinity, resulting in heteromultivalent binding or eventually even to only weak receptors98. Clearly, virus motility will enable similar recruitment of weaker or stronger receptors that by themselves also diffuse in the membrane and which ultimately needs to be incorporated into models.
Concerning protein motility in the viral envelope, current knowledge mostly derives from molecular dynamics modeling for IAV. HA and NA did not seem to rotate a lot or move over extensive distances. Instead, clumps of neighboring proteins were shown to rapidly form and dissipate while the head domains of HA and NA displayed variable tilting up to angles of 60 degrees or 90 degrees respectively99. Tilting will likely affect transfer of bound receptors from on site to another and receptor scavenging, and thereby motility.
Concluding remarks and outlook
Since the initial discovery of sialogycans as receptors for many respiratory viruses and the presence of receptor-destroying activity in several of them, research on the dynamics and complexity of virus-receptor interactions is finally coming of age. It is now evident that receptor-destruction not merely serves to facilitate release of nascent virus particles but also plays a pivotal role in the motility of virions crucial to navigate the mucosal surfaces of the respiratory tract. As a consequence receptor binding and cleavage need to be balanced to the available sialoglycome of the cell surface as well as the mucus. Adaptations in this balance are required upon host species jumps and during antigenic drift. Yet, it is also becoming clear that the complexity of these interactions is huge, not only due to enormous species-specific variation in available receptors but also in viral moieties able to interact with these receptors. This complexity is increased further by multivalency effects both from the receptor and viral point of view. Perhaps not surprisingly, modeling these interactions has not yet yielded unifying concepts. Another topic that remains to be elucidated (and was not addressed in this review) is how viruses with sialoglycan-binding properties, but lacking receptor-destroying activity navigate the respiratory tract and prevent getting stuck. These include several adenoviruses, picornaviruses, polyomaviruses and reoviruses100. Evidence is evolving for such viruses that, in addition to interactions with Sia, specific protein receptors are essential to establish binding and cell entry101,102. Possibly, Sia moieties only make a minor contribution to the binding avidity of such viruses. In the absence of a specific protein receptor in the mucus layer, the binding avidity provided by Sias will be low enough to result in short-lived mucus-virus particle interaction in order to maintain motility. Clearly, it will be important to fully unravel virus-sialoglycan interactions to understand the rules of engagement for virion motility and the consequences thereof for host tropism, pathogenesis and virus evolution.
Data availability
No datasets were generated or analyzed during the current study.
References
Zanin, M., Baviskar, P., Webster, R. & Webby, R. The interaction between respiratory pathogens and mucus. Cell Host Microbe 19, 159–168 (2016).
Wallace, L. E., Liu, M., van Kuppeveld, F. J. M., de Vries, E. & de Haan, C. A. M. Respiratory mucus as a virus-host range determinant. Trends Microbiol 29, 983–992 (2021).
Alford, R. H., Kasel, J. A., Gerone, P. J. & Knight, V. Human influenza resulting from aerosol inhalation. Proc. Soc. Exp. Biol. Med. 122, 800–804 (1966).
Wang, C. C. et al. Airborne transmission of respiratory viruses. Science 373, eabd9149 (2021).
Edinger, T. O., Pohl, M. O. & Stertz, S. Entry of influenza A virus: host factors and antiviral targets. J. Gen. Virol. 95, 263–277 (2014).
Rust, M. J., Lakadamyali, M., Zhang, F. & Zhuang, X. Assembly of endocytic machinery around individual influenza viruses during viral entry. Nat. Struct. Mol. Biol. 11, 567–573 (2004).
Navaratnarajah, C. K., Generous, A. R., Yousaf, I. & Cattaneo, R. Receptor-mediated cell entry of paramyxoviruses: Mechanisms, and consequences for tropism and pathogenesis. J. Biol. Chem 295, 2771–2786 (2020).
Thibault, P. A., Watkinson, R. E., Moreira-Soto, A., Drexler, J. F. & Lee, B. Zoonotic potential of emerging paramyxoviruses: knowns and unknowns. Adv. Virus Res. 98, 1–55 (2017).
Sriwilaijaroen, N. & Suzuki, Y. Roles of sialyl glycans in HCoV-OC43, HCoV-HKU1, MERS-CoV and SARS-CoV-2 infections. Methods Mol. Biol. 2556, 243–271 (2022).
Hulswit, R. J. G., de Haan, C. A. M. & Bosch, B.-J. Coronavirus spike protein and tropism changes. Adv. Virus Res. 96, 29–57 (2016).
Saunders, N. et al. TMPRSS2 is a functional receptor for human coronavirus HKU1. Nature 624, 207–214 (2023).
Pronker, M. F. et al. Sialoglycan binding triggers spike opening in a human coronavirus. Nature 624, 201–206 (2023).
Karakus, U. et al. MHC class II proteins mediate sialic acid independent entry of human and avian H2N2 influenza A viruses. Nat. Microbiol. 9, 2626–2641 (2024).
Karakus, U. et al. H19 influenza A virus exhibits species-specific MHC class II receptor usage. Cell Host Microbe 32, 1089–1102.e10 (2024).
Karakus, U., Pohl, M. O. & Stertz, S. Breaking the convention: sialoglycan variants, coreceptors, and alternative receptors for influenza A virus entry. J. Virol. 94, e01357–19 (2020).
Giotis, E. S. et al. Entry of the bat influenza H17N10 virus into mammalian cells is enabled by the MHC class II HLA-DR receptor. Nat. Microbiol. 4, 2035–2038 (2019).
Karakus, U. et al. MHC class II proteins mediate cross-species entry of bat influenza viruses. Nature 567, 109–112 (2019).
Balliet, J. W. et al. Production and characterization of a soluble, active form of Tva, the subgroup A avian sarcoma and leukosis virus receptor. J. Virol. 73, 3054–3061 (1999).
Shum, M. H.-H. et al. Binding affinity between coronavirus spike protein and human ACE2 receptor. Comput Struct. Biotechnol. J 23, 759–770 (2024).
Tortorici, M. A. et al. Structural basis for human coronavirus attachment to sialic acid receptors. Nat. Struct. Mol. Biol. 26, 481–489 (2019).
Fei, Y. et al. Characterization of receptor binding profiles of influenza A viruses using an ellipsometry-based label-free glycan microarray assay platform. Biomolecules 5, 1480–1498 (2015).
Sauter, N. K. et al. Hemagglutinins from two influenza virus variants bind to sialic acid derivatives with millimolar dissociation constants: a 500-MHz proton nuclear magnetic resonance study. Biochemistry 28, 8388–8396 (1989).
Tappert, M. M., Porterfield, J. Z., Mehta-D’Souza, P., Gulati, S. & Air, G. M. Quantitative comparison of human parainfluenza virus hemagglutinin-neuraminidase receptor binding and receptor cleavage. J. Virol. 87, 8962–8970 (2013).
Neu, U., Woellner, K., Gauglitz, G. & Stehle, T. Structural basis of GM1 ganglioside recognition by simian virus 40. Proc. Natl. Acad. Sci. USA 105, 5219–5224 (2008).
Burmeister, W. P., Guilligay, D., Cusack, S., Wadell, G. & Arnberg, N. Crystal structure of species D adenovirus fiber knobs and their sialic acid binding sites. J. Virol. 78, 7727–7736 (2004).
Cohen, M. Notable aspects of glycan-protein interactions. Biomolecules 5, 2056–2072 (2015).
Raman, R., Tharakaraman, K., Sasisekharan, V. & Sasisekharan, R. Glycan-protein interactions in viral pathogenesis. Curr. Opin. Struct. Biol. 40, 153–162 (2016).
Neu, U., Bauer, J. & Stehle, T. Viruses and sialic acids: rules of engagement. Curr. Opin. Struct. Biol. 21, 610–618 (2011).
Overeem, N. J., van der Vries, E. & Huskens, J. A dynamic, supramolecular view on the multivalent interaction between influenza virus and host cell. Small 17, e2007214 (2021).
Liu, M. et al. Human-type sialic acid receptors contribute to avian influenza A virus binding and entry by hetero-multivalent interactions. Nat. Commun. 13, 4054 (2022).
Lakadamyali, M., Rust, M. J. & Zhuang, X. Endocytosis of influenza viruses. Microbes Infect 6, 929–936 (2004).
de Vries, E., Du, W., Guo, H. & de Haan, C. A. M. Influenza A virus hemagglutinin-neuraminidase-receptor balance: preserving virus motility. Trends Microbiol 28, 57–67 (2020).
Sempere Borau, M. & Stertz, S. Entry of influenza A virus into host cells - recent progress and remaining challenges. Curr. Opin. Virol. 48, 23–29 (2021).
Hirst, G. K. The relationship of the receptors of a new strain of virus to those of the mumps-NDV-influenza. group. J. Exp. Med. 91, 177–184 (1950).
Palese, P., Tobita, K., Ueda, M. & Compans, R. W. Characterization of temperature sensitive influenza virus mutants defective in neuraminidase. Virology 61, 397–410 (1974).
Matrosovich, M. N., Matrosovich, T. Y., Gray, T., Roberts, N. A. & Klenk, H.-D. Neuraminidase is important for the initiation of influenza virus infection in human airway epithelium. J. Virol. 78, 12665–12667 (2004).
Sakai, T., Nishimura, S. I., Naito, T. & Saito, M. Influenza A virus hemagglutinin and neuraminidase act as novel motile machinery. Sci. Rep. 7, 45043 (2017).
Guo, H. et al. Kinetic analysis of the influenza A virus HA/NA balance reveals contribution of NA to virus-receptor binding and NA-dependent rolling on receptor-containing surfaces. PLoS Pathog 14, e1007233 (2018).
Sakai, T., Takagi, H., Muraki, Y. & Saito, M. Unique directional motility of influenza C virus controlled by its filamentous morphology and short-range motions. J. Virol. 92, e01522–17 (2018).
Wu, X. et al. Kinetic analysis of paramyxovirus-sialoglycan receptor interactions reveals virion motility. PLoS Pathog 19, e1011273 (2023).
Lewis, A. L. et al. Sialic Acids and Other Nonulosonic Acids. in: Essentials of Glycobiology (eds. Varki, A. et al.) (Cold Spring Harbor Laboratory Press, 2022).
Matrosovich, M., Herrler, G. & Klenk, H. D. Sialic acid receptors of viruses. Top. Curr. Chem. 367, 1–28 (2015).
Thompson, A. J. & Paulson, J. C. Adaptation of influenza viruses to human airway receptors. J. Biol. Chem 296, 100017 (2021).
Villar, E. & Barroso, I. M. Role of sialic acid-containing molecules in paramyxovirus entry into the host cell: a minireview. Glycoconj. J. 23, 5–17 (2006).
de Groot, R. J. Structure, function and evolution of the hemagglutinin-esterase proteins of corona- and toroviruses. Glycoconj. J. 23, 59–72 (2006).
Schjoldager, K. T., Narimatsu, Y., Joshi, H. J. & Clausen, H. Global view of human protein glycosylation pathways and functions. Nat. Rev. Mol. Cell Biol. 21, 729–749 (2020).
Narimatsu, Y. et al. An atlas of human glycosylation pathways enables display of the human glycome by gene engineered cells. Mol. Cell 75, 394–407.e5 (2019).
Zeng, Q., Langereis, M. A., van Vliet, A. L. W., Huizinga, E. G. & de Groot, R. J. Structure of coronavirus hemagglutinin-esterase offers insight into corona and influenza virus evolution. Proc. Natl. Acad. Sci. USA 105, 9065–9069 (2008).
Müller, A. et al. Structural and functional analysis of the hemagglutinin-esterase of infectious salmon anaemia virus. Virus Res 151, 131–141 (2010).
Aspehaug, V., Mikalsen, A. B., Snow, M., Biering, E. & Villoing, S. Characterization of the infectious salmon anemia virus fusion protein. J. Virol. 79, 12544–12553 (2005).
Higa, H. H., Rogers, G. N. & Paulson, J. C. Influenza virus hemagglutinins differentiate between receptor determinants bearing N-acetyl-, N-glycollyl-, and N,O-diacetylneuraminic acids. Virology 144, 279–282 (1985).
Sauter, N. K. et al. Crystallographic detection of a second ligand binding site in influenza virus hemagglutinin. Proc. Natl. Acad. Sci. USA 89, 324–328 (1992).
Zaitsev, V. et al. Second sialic acid binding site in Newcastle disease virus hemagglutinin-neuraminidase: implications for fusion. J. Virol. 78, 3733–3741 (2004).
Laver, W. G., Colman, P. M., Webster, R. G., Hinshaw, V. S. & Air, G. M. Influenza virus neuraminidase with hemagglutinin activity. Virology 137, 314–323 (1984).
Uhlendorff, J., Matrosovich, T., Klenk, H.-D. & Matrosovich, M. Functional significance of the hemadsorption activity of influenza virus neuraminidase and its alteration in pandemic viruses. Arch. Virol. 154, 945–957 (2009).
Varghese, J. N. et al. Structural evidence for a second sialic acid binding site in avian influenza virus neuraminidases. Proc. Natl. Acad. Sci. USA 94, 11808–11812 (1997).
Bakkers, M. J. G. et al. Betacoronavirus adaptation to humans involved progressive loss of Hemagglutinin-Esterase lectin activity. Cell Host Microbe 21, 356–366 (2017).
de Vries, E. & de Haan, C. A. Letter to the editor: Highly pathogenic influenza A(H5N1) viruses in farmed mink outbreak contain a disrupted second sialic acid binding site in neuraminidase, similar to human influenza A viruses. Eur. Surveill. 28, 2300085 (2023).
Greengard, O., Poltoratskaia, N., Leikina, E., Zimmerberg, J. & Moscona, A. The anti-influenza virus agent 4-GU-DANA (zanamivir) inhibits cell fusion mediated by human parainfluenza virus and influenza virus HA. J. Virol. 74, 11108–11114 (2000).
Ruiz, P. A. S., Ziebert, F. & Kulić, I. M. Physics of self-rolling viruses. Phys. Rev. E 105, 054411 (2022).
Ziebert, F. & Kulić, I. M. How influenza’s spike motor works. Phys. Rev. Lett. 126, 218101 (2021).
Agarwal, S., Veytsman, B., Fletcher, D. A. & Huber, G. Kinetics and optimality of influenza A virus locomotion. Phys. Rev. Lett. 133, 248402 (2024).
Ziebert, F., Dokonon, K. G. & Kulić, I. M. Reshaping and enzymatic activity may allow viruses to move through the mucus. Soft Matter 20, 7185–7198 (2024).
Yoshida, A. et al. Enhanced visualization of influenza A virus entry using virus-view atomic force microscopy. Preprint at https://doi.org/10.1101/2024.07.19.603848 (2024).
Müller, M., Lauster, D., Wildenauer, H. H. K., Herrmann, A. & Block, S. Mobility-Based quantification of multivalent virus-receptor interactions: new insights into influenza A virus binding mode. Nano Lett 19, 1875–1882 (2019).
Liu, M. et al. H3N2 influenza A virus gradually adapts to human-type receptor binding and entry specificity after the start of the 1968 pandemic. Proc. Natl. Acad. Sci. USA 120, e2304992120 (2023).
Sieben, C. et al. Influenza virus binds its host cell using multiple dynamic interactions. Proc. Natl. Acad. Sci. USA 109, 13626–13631 (2012).
Lam, A. et al. Single-virus assay reveals membrane determinants and mechanistic features of Sendai virus binding. Biophys. J. 121, 956–965 (2022).
Dadonaite, B., Vijayakrishnan, S., Fodor, E., Bhella, D. & Hutchinson, E. C. Filamentous. influenza viruses. J. Gen. Virol. 97, 1755–1764 (2016).
Loney, C., Mottet-Osman, G., Roux, L. & Bhella, D. Paramyxovirus ultrastructure and genome packaging: cryo-electron tomography of sendai virus. J. Virol. 83, 8191–8197 (2009).
Vahey, M. D. & Fletcher, D. A. Influenza A virus surface proteins are organized to help penetrate host mucus. Elife 8, e43764 (2019).
Lam, A., Yuan, D. S., Ahmed, S. H. & Rawle, R. J. Viral size modulates sendai virus binding to cholesterol-stabilized receptor nanoclusters. J. Phys. Chem. B 126, 6802–6810 (2022).
Benton, D. J., Martin, S. R., Wharton, S. A. & McCauley, J. W. Biophysical measurement of the balance of influenza a hemagglutinin and neuraminidase activities. J. Biol. Chem 290, 6516–6521 (2015).
Hamming, P. H. E. et al. Receptor density-dependent motility of influenza virus particles on surface gradients. ACS Appl. Mater. Interfaces 15, 25066–25076 (2023).
Yang, X. et al. A beneficiary role for neuraminidase in influenza virus penetration through the respiratory mucus. PLoS One 9, e110026 (2014).
Cohen, M. et al. Influenza A penetrates host mucus by cleaving sialic acids with neuraminidase. Virol. J. 10, 321 (2013).
Liu, M., van Kuppeveld, F. J., de Haan, C. A. & de Vries, E. Gradual adaptation of animal influenza A viruses to human-type sialic acid receptors. Curr. Opin. Virol. 60, 101314 (2023).
Hall, C. B. Respiratory syncytial virus and parainfluenza virus. N. Engl. J. Med 344, 1917–1928 (2001).
Korosec, C. S. et al. Motility of an autonomous protein-based artificial motor that operates via a burnt-bridge principle. Nat. Commun. 15, 1511 (2024).
Ait-Haddou, R. & Herzog, W. Brownian ratchet models of molecular motors. Cell Biochem Biophys 38, 191–214 (2003).
Xiong, X. et al. Receptor binding by a ferret-transmissible H5 avian influenza virus. Nature 497, 392–396 (2013).
Walther, T. et al. Glycomic analysis of human respiratory tract tissues and correlation with influenza virus infection. PLoS Pathog 9, e1003223 (2013).
Gulati, S. et al. Human H3N2 influenza viruses isolated from 1968 To 2012 show varying preference for receptor substructures with no apparent consequences for disease or spread. PLoS One 8, e66325 (2013).
Chan, R. W. Y., Chan, M. C. W., Nicholls, J. M. & Malik Peiris, J. S. Use of ex vivo and in vitro cultures of the human respiratory tract to study the tropism and host responses of highly pathogenic avian influenza A (H5N1) and other influenza viruses. Virus Res 178, 133–145 (2013).
Kumari, K. et al. Receptor binding specificity of recent human H3N2 influenza viruses. Virol. J. 4, 42 (2007).
Bradley, K. C. et al. Analysis of influenza virus hemagglutinin receptor binding mutants with limited receptor recognition properties and conditional replication characteristics. J. Virol. 85, 12387–12398 (2011).
Wagner, R., Matrosovich, M. & Klenk, H.-D. Functional balance between haemagglutinin and neuraminidase in influenza virus infections. Rev. Med. Virol. 12, 159–166 (2002).
Lang, Y. et al. Coronavirus hemagglutinin-esterase and spike proteins coevolve for functional balance and optimal virion avidity. Proc. Natl. Acad. Sci. USA 117, 25759–25770 (2020).
Gui, L. et al. Electron tomography imaging of surface glycoproteins on human parainfluenza virus 3: association of receptor binding and fusion proteins before receptor engagement. mBio 6, e02393–02314 (2015).
Bárcena, M. et al. Cryo-electron tomography of mouse hepatitis virus: Insights into the structure of the coronavirion. Proc. Natl. Acad. Sci. USA 106, 582–587 (2009).
Mögling, R. et al. Neuraminidase-mediated haemagglutination of recent human influenza A(H3N2) viruses is determined by arginine 150 flanking the neuraminidase catalytic site. J. Gen. Virol 98, 1274–1281 (2017).
Benton, D. J., Wharton, S. A., Martin, S. R. & McCauley, J. W. Role of neuraminidase in influenza A(H7N9) virus receptor binding. J. Virol. 91, e02293–16 (2017).
Chlanda, P. et al. Structural analysis of the roles of influenza A virus membrane-associated proteins in assembly and morphology. J. Virol. 89, 8957–8966 (2015).
Harris, A. et al. Influenza virus pleiomorphy characterized by cryoelectron tomography. Proc. Natl. Acad. Sci. USA 103, 19123–19127 (2006).
Tang, B. et al. Sphingomyelin-sequestered cholesterol domain recruits formin-binding protein 17 for constricting clathrin-coated pits in influenza virus entry. J. Virol. 96, e0181321 (2022).
Sieben, C., Sezgin, E., Eggeling, C. & Manley, S. Influenza A viruses use multivalent sialic acid clusters for cell binding and receptor activation. PLoS Pathog 16, e1008656 (2020).
Huskens, J. et al. A model for describing the thermodynamics of multivalent host-guest interactions at interfaces. J. Am. Chem. Soc 126, 6784–6797 (2004).
Choi, H.-K., Lee, D., Singla, A., Kwon, J. S.-I. & Wu, H.-J. The influence of heteromultivalency on lectin-glycan binding behavior. Glycobiology 29, 397–408 (2019).
Casalino, L. et al. Breathing and tilting: mesoscale simulations illuminate influenza glycoprotein vulnerabilities. ACS Cent. Sci. 8, 1646–1663 (2022).
Blaum, B. S. & Stehle, T. Sialic acids in nonenveloped virus infections. Adv. Carbohydr. Chem. Biochem. 76, 65–111 (2019).
Varanese, L. et al. MFSD6 is an entry receptor for enterovirus D68. Nature 641, 1268–1275 (2025).
Koehler, M. et al. Glycan-mediated enhancement of reovirus receptor binding. Nat. Commun. 10, 4460 (2019).
Liu, D. X., Liang, J. Q. & Fung, T. S. Human Coronavirus-229E, -OC43, -NL63, and -HKU1 (Coronaviridae). Encyclopedia Virol. 428–440 https://doi.org/10.1016/B978-0-12-809633-8.21501-X (2021).
Jin, M. et al. Human coronavirus HKU1 spike structures reveal the basis for sialoglycan specificity and carbohydrate-promoted conformational changes. Nat. Commun. 16, 4158 (2025).
Huang, X. et al. Human Coronavirus HKU1 spike protein uses O-acetylated sialic acid as an attachment receptor determinant and employs hemagglutinin-esterase protein as a receptor-destroying enzyme. J. Virol. 89, 7202–7213 (2015).
Wu, X. et al. Unraveling dynamics of paramyxovirus-receptor interactions using nanoparticles displaying hemagglutinin-neuraminidase. PLoS Pathog 20, e1012371 (2024).
Rameix-Welti, M.-A., Enouf, V., Cuvelier, F., Jeannin, P. & van der Werf, S. Enzymatic properties of the neuraminidase of seasonal H1N1 influenza viruses provide insights for the emergence of natural resistance to oseltamivir. PLoS Pathog 4, e1000103 (2008).
Vajda, J., Weber, D., Brekel, D., Hundt, B. & Müller, E. Size distribution analysis of influenza virus particles using size exclusion chromatography. J. Chromatogr. A 1465, 117–125 (2016).
Calder, L. J., Wasilewski, S., Berriman, J. A. & Rosenthal, P. B. Structural organization of a filamentous influenza A virus. Proc. Natl. Acad. Sci. USA 107, 10685–10690 (2010).
Garcia, J.-M. et al. Investigation of the binding and cleavage characteristics of N1 neuraminidases from avian, seasonal, and pandemic influenza viruses using saturation transfer difference nuclear magnetic resonance. Influenza Other Respir. Viruses 8, 235–242 (2014).
Ilyushina, N. A., Bovin, N. V. & Webster, R. G. Decreased neuraminidase activity is important for the adaptation of H5N1 influenza virus to human airway epithelium. J. Virol. 86, 4724–4733 (2012).
Cabezas, J. A. et al. Neuraminidase from influenza virus A (H3N2): specificity towards several substrates and procedure of activity determination. Biochim. Biophys. Acta 616, 228–238 (1980).
Hussain, S. et al. Reduced sialidase activity of influenza A(H3N2) neuraminidase associated with positively charged amino acid substitutions. J. Gen. Virol. 102, (2021).
Halldorsson, S., Sader, K., Turner, J., Calder, L. J. & Rosenthal, P. B. In situ structure and organization of the influenza C virus surface glycoprotein. Nat. Commun. 12, 1694 (2021).
Rosenthal, P. B. et al. Structure of the haemagglutinin-esterase-fusion glycoprotein of influenza C virus. Nature 396, 92–96 (1998).
Cook, J. D., Sultana, A. & Lee, J. E. Structure of the infectious salmon anemia virus receptor complex illustrates a unique binding strategy for attachment. Proc. Natl. Acad. Sci. USA 114, E2929–E2936 (2017).
Li, Z. et al. Synthetic O-acetylated sialosides facilitate functional receptor identification for human respiratory viruses. Nat. Chem. 13, 496–503 (2021).
Langereis, M. A., Zeng, Q., Heesters, B. A., Huizinga, E. G. & de Groot, R. J. The murine coronavirus hemagglutinin-esterase receptor-binding site: a major shift in ligand specificity through modest changes in architecture. PLoS Pathog 8, e1002492 (2012).
Acknowledgements
The work performed for this review was financially supported by the International Coordination of Research on Infectious Animal Diseases (ICRAD; EPICVIR grant) and the National Institute of Allergy and Infectious Diseases, (NIAID; 75N93021C00014). The funders had no role in study design, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
E.d.V. and C.A.M.d.H conceptualized and structured the manuscript. M.L. and E.d.V. wrote the main manuscript. M.L. prepared the figures. All authors have read and approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, M., de Vries, E. & de Haan, C.A.M. Virion motility of sialoglycan-cleaving respiratory viruses. npj Viruses 3, 59 (2025). https://doi.org/10.1038/s44298-025-00140-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s44298-025-00140-x