Abstract
Although the COVID-19 pandemic is no longer a global health emergency, many patients still suffer from long-term effects, known as post-acute sequelae of COVID-19 (PASC) or long COVID. Understanding its complex pathophysiology requires animal models replicating the post-acute phase, which may aid in developing, the urgently needed, therapeutics. Our review assessed and summarized 81 studies from 1979 manuscripts. In addition, a second table summarizing the imaging findings of 26 studies related to this topic was added, based on a separate literature search of 797 manuscripts. In humans a SARS-CoV-2 infection, the sequelae and possible development of PASC is heterogenic. The same holds true for experimental animal models. While several models are suitable to address different research questions, no single model can fully replicate all aspects of PASC. Imaging plays a crucial role in visualizing these aspects, especially since questionnaires, the primary diagnostic tool in humans, cannot be used in animals. Thus, imaging allows the investigation of pathophysiology in a controlled setting, offering valuable insights. This review summarizes the available animal models and imaging modalities used in PASC research. Our aim is to provide researchers with guidance on selecting the most appropriate model and imaging technique to address their specific research questions.
Similar content being viewed by others
Introduction
While the COVID-19 pandemic, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is no longer declared as global health emergency, over 400 million patients are still not recovered1,2,3,4. They suffer from prolonged disease known as long COVID or post-acute sequelae of COVID-19 (PASC). Following the statistics of the WHO, at least 777 million individuals have been reported to be infected by SARS-CoV-2 worldwide5. As these are only the reported cases, this number is certainly an underestimation of the actual number of individuals infected by SARS-CoV-2. Of the SARS-CoV-2 infected individuals, 10-30% of the non-hospitalized cases, but even 50–70% of the hospitalized cases, develop symptoms that can be diagnosed as PASC6,7. Overall, these numbers show that although the pandemic might be over, the problems are not.
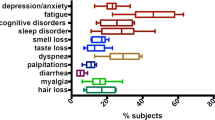
The definition of PASC is that after the initial SARS-CoV-2 infection, symptoms persist for at least three months and/or new ones have emerged and last for at least two months5. PASC has debilitating effects on patients’ quality of life and encompasses a broad range of over 200 symptoms affecting multiple organ systems, including psychological, cardiopulmonary, neurological, gastrointestinal, and ear-nose-throat issues6,8. The most frequent symptoms are extreme fatigue, post-exertional malaise (PEM), pain, and cognitive dysfunction9,10.
Five mechanisms have been hypothesized as main driving forces behind PASC, i.e., prolonged inflammation caused by viral persistence, viral-induced autoimmunity, dysfunctional neuronal signalling, alterations of the renin-angiotensin system (RAS), and microbiome dysbiosis11,12. However, it remains unclear if and how these mechanisms are interconnected and how they relate to the various pathophysiological alterations, which limit the development of diagnostic and, even more important, treatment strategies6,7,12. Animal models that mimic the post-acute phase of the infection are valuable to understand the pathophysiology of PASC, especially when combined with the clinical data from patients. Given the intricate interplay between the immune system and nervous system, it is essential to incorporate complex biological systems (e.g., animals) rather than isolated cells or samples, for assessing which pathways may contribute to the observed symptoms in patients. This approach allows for controlled conditions, accessibility to all organ systems, both cursief in a longitudinal manner via imaging and ex vivo, and with this, a comprehensive understanding of complex interactions that are difficult to study in patients7,11. Furthermore, in contrast to humans, animals can serve as their own control, which allows identification of small personalized differences between pre-infection and post-SARS-CoV-2 infection values. Combining systemic alterations derived from blood samples with local anatomical and functional changes visualized with different imaging modalities. Several animal models have proven their value in the acute phase of a SARS-CoV-2 infection, mostly studying viral pathogenesis, transmission, and vaccines13,14,15,16. The aim of this review is to provide an update on the experimental animal models used beyond the acute phase and to discuss their added value for PASC research. With the goal to give researchers guidance in selecting the model and imaging technique most suitable to answer their research question.
Methods
Literature search
A literature search in PubMed and Web of Science was performed on the 7th and 8th of October 2024 using the following search strategies: “covid” OR “sars-cov-2” AND “ long covid” OR “PASC” AND “preclinical” OR “animal” OR “mouse” OR “hamster” OR “non-human primate” OR “macaque” OR “ferret”. This research yielded 1979 abstracts, which were screened based on their title and abstract. When necessary, full manuscripts were reviewed to determine eligibility. Studies were included if they involved in vivo research using SARS-CoV-2-infected models with a follow-up period of more than 14 days post-infection. A total of 81 articles met these criteria and were summarized in Table 1.
For the table which includes imaging modalities, an additional literature search was performed in PubMed on the 17th of June 2025 using the following search strategies: “covid” OR “sars-cov-2” AND “imaging” AND “preclinical” OR “animal” OR “mouse” OR “hamster” OR “non-human primate” OR “macaque” OR “ferret”. This resulted in 797 abstracts which were screened based on title and abstract, and were necessary the entire manuscript for eligibility; articles should include in vivo imaging research with a SARS-CoV-2 infection in animals, followed for longer than 14 days post-infection. Sixteen articles fulfilled these criteria and were summarized in Table 2. Using the snowball method, 11 more articles were added.
Results
Impact on PASC development
Infection stages and severity
For humans, the acute phase lasts for two weeks, followed by a so-called post-acute phase, between 2 and 12 weeks post-infection. When patients have medically unexplained symptoms following a SARS-CoV-2 infection for at least 3 months, it is considered a chronic form of COVID-19, i.e., PASC5,7,17.
For experimental animal models, the definition of PASC is less well defined, partly because a comprehensive and continuous clinical evaluation of the infected animals is not always performed. This raises the question whether the viral kinetics between humans and experimental models are comparable. Although some differences are likely due to the high heterogeneity of the disease, the viral peak and subsequent clearance are generally similar in terms of days post-infection across all animal models (Table 3). For humans, the exact day of infection cannot be determined, but based on all the data collected so far, an average clearance time of 8–12 days is estimated. A study in which healthy human volunteers were inoculated with SARS-CoV-2 and followed for a year, which confirmed these data18,19. The peak of infection was, in general, detected a bit later in humans (day 5) compared to the various animal models (day 2–4), though the first symptoms in humans were already starting 2–4 days after inoculation. Overall, since the viral kinetics in the acute phase in the various animal models seem to be comparable to humans, it can be hypothesized that this is also the case for the timing regarding the development of PASC. However, as animals age at different rates compared to humans, it can also be hypothesized that PASC develops more rapidly in rodents than in non-human primates (NHPs)20,21. For this review, we have chosen to include studies that extend beyond the acute phase of infection, so with a minimum follow-up of 15 days.
In humans, the variant of the virus impacts the incidence of PASC, but this difference is not significant, with an incidence of 10.4% for the Wuhan variants of the virus, 9.5% for the Delta variant, and 7.8% for the Omicron variant22. The development of PASC in humans is mainly influenced by the vaccination status, with a decrease in likelihood to develop PASC after vaccination between 45 and 56% dependent on the various virus variants22.
In addition to vaccination status, disease severity in humans impacts the development of PASC, with a likelihood of PASC development in 50–70% for hospitalized patients. In hamsters, the inoculation dose was shown to have a positive correlation with disease severity23. Furthermore, in mice, it was shown that the route of infection impacts the development of disease and its pathological features24,25. In certain mouse models, aerosol infection but not intranasal infection, has the potential to induce PASC-like disease24,25. This is related to the severity of the disease in the active phase. Such large differences were not observed in NHPs, although variations were still noted between the different inoculation methods (intranasal, intratracheal, intrabronchial, intraocular, intragastric, aerosol, and mucosal exposure)26.
Since mice, compared to other animal models, lack the compatibility towards the human angiotensin converting enzyme 2 (ACE2) receptor27,28, genetic modification of mice is necessary for research using the SARS-CoV-2 virus. The advantage of genetic manipulation is that it allows for the construction of models representing all levels of severity of disease. For example, the K18 mouse expresses ACE2, which makes it susceptible to a SARS-CoV-2 infection28,29, with a relatively high mortality rate in these mice. This makes the K18 model suitable for the representation of severe, hospitalized patients, but it does not mimick the more mild disease. The hACE2/hTMPRSS2 KI mouse model, which expresses both ACE2 and human transmembrane serine protease 2 (hTMPRSS2), with the hTMPRSS2 being involved in the priming of the spike protein of SARS-CoV-2, exhibits a mild-to-moderate form of the disease30,31. For research on PASC, both mouse models are valuable, as PASC has been observed across all severity levels of COVID-19 in patients.
The influence of sex and age
In humans, there is a trend indicating that sex and age influence the development of PASC. For example, there is a statistically significant increased risk for boys up to the age of five, an increased risk of about 20% for women between 12 and 50 years of age, followed by a slightly increased risk for men between 50 and 70 years of age to develop PASC32. In children, in general, the prevalence of PASC increases with age, and 12–18-year-old adolescents are more prone to PASC than children younger than five years32,33,34,35, independent of the sex. In addition, the distribution of symptoms differs between women and men as women are more prone to neurological or neuropsychiatric symptoms related to PASC36,37. Unfortunately, there is a lack of targeted studies using animal models mimicking children, as the majority of the studies discussed in this review mainly use adult animals (Table 1). Nevertheless, differences in sex and age were still observed in several animal models. For instance, in young and adult ferrets significant differences in lung and nasal turbinate pathology and virus shedding in the upper respiratory tract were described in refs. 38,39. In NHPs, it has been shown that age has an impact on the length of viral shedding in the respiratory tract, but so far, no differences on disease outcome were reported40,41. However, it seems that there is a delay in the restoration of homeostasis in aged NHPs, due to the persistence of pro-inflammatory responses, examined till 21 dpi40.
There is a clear difference in the long-lasting effects of SARS-CoV-2 between male and female hACE2/hTMPRSS2 KI mice. In males, alterations in the lungs in the active phase were detected as well as a transient reduction in the locomotion after 6 months, these effects were not observed in females30. In ferrets, males and females showed differences in the expression of several stress response elements in the hearts42. Syrian golden hamsters show sex differences in the development of neurological symptoms, with female hamsters showing signs of depression, and males manifesting signs of anxiety43.
PASC related research area’s
Immune system
Almost all studies included in Table 1 investigated systemic alterations, predominantly focussing on the presence of antibodies or chemokines/cytokines using blood samples. For example, alterations in anti-spike protein and neutralizing antibodies were detected during the entire period for all of the experiments. In hamsters, it was also shown that reinfection revealed long-term antibody reactivity towards multiple variants of the virus, which also protected them from severe disease25,31,44. Moreover, all animal models showed an activated status of the immune system with elevated levels of cytokines like IL-6 and TNF in blood, which lasts for months after a SARS-CoV-2 infection44,45,46,47,48. A similar immune activation is also observed in human patients49.
In NHPs, changes in dendritic cell populations in the blood have been identified up to 44 dpi, accompanied by increased levels of various cytokines, granulocytes, and monocytes47,48,50. In humans, immune cell compositions and dynamics have been studies using peripheral blood mononuclear cells (PBMCs) or multi-omics approaches, showing large transcriptional and cellular changes over time. These include decreases in inflammatory monocytes and antiviral responses of innate immune cells such as dendritic cells51. Overall, there are multiple similarities detected in the responses between humans and animal models.
In addition to systemic immune alterations, primarily investigated through blood samples, local immune changes have also been assessed in vivo using bronchoalveolar lavage (BAL) fluid in twelve studies summarized in Table 1. These studies were conducted predominantly in NHPs, but also in mice and ferrets. Three of the studies38,52,53 performed BAL sampling at the study endpoint, despite evidence that BAL procedures can influence lung appearance on computed tomography (CT) imaging and gross pathology, particularly within the first 24 h post-procedure54. BAL fluid analyses revealed various immune markers indicative of an early local immune response. Notably, natural killer (NK) cells appeared to play a crucial role in modulating alveolar macrophage function and influencing viral persistence in the lungs40,55,56,57. Transcriptional profiling of BAL fluid56 demonstrated local immune signatures distinct from those of PBMCs, highlighting a unique role for IL-10 in memory cell formation and suppression of specific T-cell subsets. Furthermore, age-related differences in BAL fluid, including altered DNase I activity were reported38 while gene expression analyses58 showed marked differences between vaccinated and unvaccinated animals. Perhaps the most striking finding, however, is the detection of replication-competent SARS-CoV-2 in BAL macrophages as late as six months post-infection55, suggesting long-term viral persistence in the lung microenvironment.
Blood circulation
Disruptions in the blood circulation due to SARS-CoV-2 infection may have an impact on the entire body, including endothelial function, changes in vascular density, and the formation of microclots. Furthermore, the size and stiffness of blood cells change and may affect oxygen delivery to tissues6. Alterations in blood are detected in all animal models38,42,48,50,59,60,61. Though it is unclear whether these changes are related to the animals’ recovery from the acute infection or if they represent chronic alterations associated with a form of PASC, as most of them are detected in the first months post-infection.
In hamsters, followed untill 20 dpi, changes were observed in markers of renal disease, blood lipids, and energy-related markers. Additionally, neutrophil extracellular traps (NETs) were identified in the vasculature of the lungs at an early stage after infection. While NETs can cause vasculitis or vascular barrier injury, these lesions were largely resolved by 14 dpi62. In mice, NETs were also detected alongside neutrophil dysregulation, persisting up to 30 dpi59.
In contrast, ferrets show no signs of macroscopic or microscopic vascular thrombosis in any part of the lungs, and plasma coagulation factors showed only a minimal variation after infection63. Similar findings were reported before, which demonstrated that differences in age were a determinant for the formation of NETs, but without a strong clinical phenotype38. Nevertheless, ferrets show SARS-CoV-2-induced changes in the heart-related stress response elements. This suggests that even during a mild disease, alterations are induced already at 14 dpi38,42.
Central nervous system
Persistent neurological symptoms associated with SARS-CoV-2 have drawn significant attention since the onset of the pandemic. These symptoms, among the most common features of PASC, cover a wide range and include fatigue, headaches, loss of taste and smell, sleep disturbances, and cognitive symptoms such as concentration and memory problems, often described as “brain fog”64. The precise route and mechanism by which and if SARS-CoV-2 enters the brain remains unclear. Evidence suggests that SARS-CoV-2 can invade the central nervous system (CNS), as it has been detected in post-mortem brain tissue from deceased COVID-19 patients65,66,67. However, there is lack of evidence for replicating virus in the brain, and the presence of SARS-CoV-2 antigens in brain tissue samples is only confirmed in few studies67,68,69. Moreover, no evidence of viral presence has been reported in brains of PASC patients. Various animal models have also demonstrated the neuroinvasive properties of SARS-CoV-2 with the detection of viral RNA by PCR, which does not indicate the presence of replicative virus or simply circulating RNA fragments25,70,71,72,73,74,75. Only one study in hamsters detected SARS-CoV-2 RNA in the brainstem 80 dpi, and also isolated replicative virus, suggesting viral persistence in the brain43.
Fatigue
One of the most common symptoms in PASC is fatigue, which can be worsened by minor physical or cognitive exertion, leading to PEM. This is reported in about 25% of the affected individuals76. In addition, it is the most narrative parameter for recovery of patients themselves. The measurement of fatigue in animals is complicated. Locomotor function can be relatively easy determined, but most animals hide weaknesses due to their survival instinct. Furthermore, exertion can be both physical and/or cognitive6,76,77. It often consists of multiple components and is also influenced by individual behavior, which makes it challenging to achieve specificity for the target78. Behavior decline can be assessed in animal models, but this is a challenging process even when the animal serves as its own control.
Neuroinflammation
Transcriptomic analysis of brains from k18-hACE2 mice 1 month and 4 months post-infection suggested activation of immune pathways such as complement activation and phagocytosis recognition. However, minimal microglia and astrocyte activation was reported in these mice in the post-acute time points, suggesting no active viral replication in the post-acute phase79. Behavior analyses in these mice suggested development of motor dysfunction, including ataxia, and an impaired neuropsychiatric state.
In human ACE2 transgenic mice and hamsters, meningoencephalitis increased activation of microglia and a reduction in dendritic spine density have been reported80,81,82,83. In humans also glial fibrillary acidic protein (GFAP) in blood and neurological markers, like tau, are detected, suggesting ongoing neuroinflammation84.
NHPs have shown, with in vivo imaging, increased expression of translocator protein (TSPO) across the brain, a marker of neuroinflammation also seen in brains of patients with PASC47,85. In addition, in post-mortem analysis, microhemorrhages and brain hypoxia have been observed in NHPs, including alterations in the astrocytes and microglia70.
Furthermore, it has been hypothesized that a SARS-CoV-2 infection may contribute to neurodegeneration and protein aggregation. A number of signalling pathways associated with neurodegenerative diseases, including Alzheimer’s disease (AD)86 and Parkinson’s disease (PD)87, have been found to be affected in the brains of patients with PASC. A defining feature, for instance, for Parkinson’s disease is the formation of Lewy bodies, which are intracellular accumulations of α-synuclein. Interestingly, such α-synuclein accumulations have also been identified in hamsters (21 dpi) and NHPs (35–42 dpi) infected with SARS-CoV-282,88. A transcriptomic analysis performed on the brainstem of Syrian golden hamsters revealed a neurodegenerative signature at 80 dpi. This signature includes transcriptional changes in the proteasome pathway, oxidative phosphorylation, and dopaminergic and glutamatergic synapses43.
In patients with PASC, a broad range of emotional and psychiatric problems, including depression, anxiety, mood changes, and cognitive impairments, have been reported89. In general, behavioral tests can be used to assess symptoms that are not directly detectable via clinical observation in humans, but also in animals. For instance, persistent abnormalities in neuropsychiatric state and behavior in SARS-CoV-2 infected mice were identified by a standardized set of experiments measuring the phenotype (the SmithKline Beecham, Harwell, Imperial College, Royal London Hospital, Phenotype Assessment (SHIRPA))30,90. Only one of those study found that the motor behavior via the SHIRPA test of infected mice stayed impaired or even got worse over a 4-month period post-infection, while this was not determined by the other study30,90.
In SARS-CoV-2-infected hamsters, different behavioural tests have been used to study the effect of SARS-CoV-2 infection beyond the acute phase. Anxiety-like behavior was observed using the marble burying test and the novelty-suppressed feeding test at 26 dpi91. Depression-like features were also found in female hamsters using the splash test at 80 dpi43, and signs of impaired short-term memory during infection were demonstrated using the object recognition test at 80 dpi43.
The mechanisms underlying all of the above mentioned symptoms remain poorly understood and it is unclear if they results from direct effects of parts of the virus (mRNA or peptides), viral persistence in the brain or that it is due to indirect effects caused by disturbances in the immune system or vasculature.
Metabolism
Several symptoms of PASC have been linked to metabolic abnormalities, including impaired energy production, highlighting the relevance of metabolic dysregulation in long COVID research. Deviations in cellular metabolic patterns can affect multiple physiological pathways and have previously been observed in the pathophysiology of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS)92,93. To date, only a limited number of animal studies have addressed these metabolic manifestations. In mice and hamsters, investigations have focused primarily on obesity, reporting delayed recovery and increased incidence of cardiomyopathy in obese animals compared to lean controls94,95. In NHPs, a SARS-CoV-2 infection has been shown to alter the composition and functional activity of the gut microbiota96. Another study identified hyperglycemia following infection in both humans and animals, suggesting a potential association97. Glucose homeostasis is primarily maintained by the liver and gut microbiota, linking findings from both studies. Baindara et al. 97 propose that hyperglycemia may play a central role in facilitating SARS-CoV-2 entry into host cells. In the acute phase of COVID-19, hyperglycemia has been associated with increased disease severity98. In NHPs, both hyperglycemia, measured using [18F]FDG PET-CT, and alterations in the gut microbiota have been observed following infection96,97, suggesting that similar metabolic mechanisms may be affected in both NHPs and humans. Whether these mechanisms are conserved across other animal models remains to be determined.
Organ and bone, cartilage, muscle
Organ impairment
The primary target organ of a SARS-CoV-2 infection is the respiratory tract. Fibrosis in all types of tissues is also found in patients with PASC. As described in the studies in Table 1, the research into fibrosis in animals is focused on the lungs. Although medical imaging suggests that the lungs of both mice and hamsters recover after the active phase, detailed ex vivo analyses have revealed fibrosis formation at 20 and 120 dpi, resembling what is observed in humans60,99,100.
Damage to other organs in the body than the lungs is less clear and appears to result more from immune system activation and the inflammatory response rather than direct by the virus6. However, in about 30% of patients, some form of renal dysfunction has been found after an infection, which is most often related to a form of fibrosis101. In a mouse surrogate model for SARS-CoV-2 using MHV-1, similar alterations are observed, which suggests the possible development of renal fibrosis101. Furthermore, SARS-CoV-2-infected mice and hamsters show fibrosis formation in the heart, probably as a direct consequence of the infection.95,102. The SARS-CoV-2 spike protein induces alterations in multiple physiological processes in the body, one of these being the disturbance of (mitochondrial) metabolic genes that can facilitate fibrosis in multiple organs95. A corresponding marker for this process has been identified in both hamsters infected with SARS-CoV-2 and patients with confirmed PASC102. In general, fibrosis formation after a SARS-CoV-2 infection is described in multiple mouse models and hamsters, but less in NHPs95,99,100,101,102.
Bone and muscle impairment
In general, it is known that inflammatory diseases of the respiratory tract have an impact on bone metabolism. Among the patients with severe acute COVID-19, around 24% of the patients with PASC showed skeletal pathologies103. Directly related to these pathologies are the skeletal muscle weakness and exercise intolerance found in PASC patients104,105. This muscle weakness has not only an impact on the quality of life of patients but also seems to be correlated to metabolic alterations. In hamsters, SARS-CoV-2 induced bone loss and inflammation, together with an increased risk of arthritis103,106. However, experiments to test muscle weakness or visualize this process via imaging in experimental models are scarce, probably due to a lack of dedicated imaging tracers. In Syrian golden hamsters infected with SARS-CoV-2, bone and cartilage damage in the proximal epiphysis of the tibia was identified using histological micro-computed tomography (μCT) imaging. To a certain extent, this might mimick the reactive arthritis and joint pain observed in PASC patients10,106,107. Similar damage has been reported in the lumbar region and femur of SARS-CoV-2 infected hamsters using μCT imaging60,103.
Imaging
In several animal studies, in vivo imaging was applied as translational modality and read-out parameter for both anatomical and functional alterations. These articles are summarized in Table 2. Medical imaging remains a critical tool for diagnosing and assessing COVID-19 patients, particularly in the evaluation of lung pathology. In patients with PASC, several studies have investigated the persistence of lung lesions and inflammation after viral clearance, utilizing different imaging techniques49,108,109,110. However, the role of medical imaging in understanding the effects of PASC on other organs is growing, as more emphasis now also lies on brain imaging and total body assessments. Notwithstanding, still relatively few clinical studies have examined the long-term consequences in living PASC patients with the aid of imaging.
Lungs
While human imaging studies into PASC seem to shift focus to multiple organ system assessment, most animal models focus on the long-term effects on lung tissue30,40,48,53,57,111,112,113,114,115,116,117,118,119,120,121,122,123. For instance, in hACE2/hTMPRSS2 KI mice, impaired lung function, including increased pulmonary inflammation and decreased functional tidal volume, has been observed by μCT30. In NHPs, already since the beginning of the pandemic, most studies have focused on radiographs and CTs to complement virologic assessments with meaningful translational read-outs, as the lung imaging features found in humans are closely mirrored in NHPs26,124. More recently, studies with different PET tracers were published, allowing functional imaging to provide knowledge regarding the pathophysiology of the SARS-CoV-2 infection and activation of (immune-related) processes in the body47,48,56,88,112,113,124,125,126,127,128,129. The studies, using different tracers than the wide-spread used [18F]FDG, revealed widespread inflammation in the anatomically unaffected lung tissue, presence of the spike protein in lung lesions and more prominent uptake of pro-inflammatoir monocytes in the lung draining lymph nodes compared to the lesions in the lungs but also viral detection in various other organs besides the respiratory tract47,48,113,124,125,126,127.
Brain
For neuro-imaging in PASC patients, studies with different imaging modalities have been conducted. These are described in detail in another review, which highlights that these imaging modalities can also be applied in animal models to study disease processes in a more controlled environment130. In vivo imaging of the brain in animal models has so far been limited to a few NHP studies using different PET-tracers. Whereas [18F]FDG-PET studies in human PASC patients showed hypometabolism in various brain regions131 results in a NHP study only showed increased tracer uptake in the pituitary gland of 2 out of 8 animals up to 35 dpi88. This difference may be due to the difference in time post-infection, as [18F]FDG PET can be used to visualize the potential transition from inflammatory hypermetabolism during the first period of neuroinflammatory activity (as may have been observed in NHP’s 35 dpi) to hypometabolic dysfunctions due to chronic neuroinflammatory activity (as observed in human PASC patients often several months to even years after infection)49,131,132. This stresses the need for longer follow-up period in PASC research with animal models, to allow for more direct translation of findings to human PASC findings. Another NHP study using [18F]DPA-714, as a PET tracer for neuroinflammation, showed tracer uptake throughout the brain in all animals up to 44 dpi47. These results correspond to observations in humans with PASC47,85. Though magnetic resonance imaging (MRI) has shown changes in brain structure associated with SARS-CoV-2 in humans133, only one article was found using this type of imaging in animal models134. In this manuscript, an array of imaging-related alterations was detected characteristic of cerebral small vessel disease, which persisted for at least 7 months post-infection. While another closely related imaging technique, magnetoencephalography (MEG), showed preservation of neural activity in certain brain regions in vaccinated animals compared to controls135.
Whole body
In addition to the lungs and the brain, several studies, exclusively in NHPs, performed whole body imaging56,124,127,129. These studies demonstrated that multiple body parts can be affected following a SARS-CoV-2 infection, or that systemic immune activation may occur. Two of these studies also tracked the viral dissemination throughout the body with a dedicated PET-tracer targeted towards the virus125,127. One study focused on the acute phase only125, while the other performed extended observations for over 3 months127. Both studies showed increased signal in multiple anatomical regions.
Two other studies focused on specific organs besides the brain and lungs. One case study described increased uptake of [18F]FDG in parts of the intestine, which was confirmed by pathology and anal swabs128. The other study, uniquely using ultrasound imaging, evaluated the cardiac function after a SARS-CoV-2 infection in mice136. At 24 weeks post-infection, a significant decrease in cardiac ejection fraction was observed via echocardiography. Additionally, a significant increase in the left ventricular end-systolic diameter was noted, indicating reduced myocardial contractility and consequent cardiac damage136.
Animal models for PASC
Overall, several animal models have been tested and validated to understand the pathogenesis of SARS-CoV-2 and to allow evaluation of predominantly newly developed vaccines but also treatment opportunities in the acute phase27. All models had their own advantages and disadvantages during this initial stage of research137,138. Many of these models are also contributing to the research performed after the acute phase of a SARS-CoV-2 infection. The alterations shown in patients with PASC are not detected in all patients and also not in all animals. Due to the heterogeneity in symptoms, patients might be categorized in different phenotypes based on symptoms (e.g., with PEM or without) and/or molecular signature (e.g., altered protein expression). The likelihood that only one animal model can mimic the complete and highly heterogeneous human phenotype of PASC is low. This raises the question what the translational characteristics for each model for PASC are and in which way experimental models could most optimally be employed for a certain PASC phenotype7,11. Mice, hamsters, and NHPs are the most commonly used animal models for PASC, and their key characteristics are summarized in Table 4.
Artificial mouse models
A different approach to study PASC is the use of artificial models where material from patients is transferred to animals. A relatively short time after transplantation, the animals can be checked for the development of symptoms, and afterwards, the testing of therapies. With this idea in mind, mice received a fecal transplant of confirmed COVID-19 patients with or without symptoms and healthy naïve controls. Besides alterations in the gut microbiota of the mice, the mice receiving microbiota from confirmed PASC patients also showed impaired cognitive performance and pulmonary tissue damage139. This suggests that SARS-CoV-2 induces alterations in the microbiota that drive several symptoms. Something similar is described with the passive transfer of total IgG, to study whether autoantibodies from PASC patients could trigger symptoms in mice140,141. Plasma biomarker analysis revealed three subgroups of PASC patients, based on changes in markers linked to neuronal damage, astrocyte activation, and IFN-signature. The subgroup of mice with higher IFN-signature showed reduced locomotor activity without affecting their motor coordination. IgG transfer also induced pronounced and persistent sensory hypersensitivity in two different independent studies. Autoantibodies analysis showed increased autoreactivity to neurological proteins and to common autoantigens141. Those results suggest that autoantibodies could have a causative role in the pathogenesis of PASC140,141. A slightly different approach is used with the infusion of the SARS-CoV-2 spike protein directly into the brains of mice via an intercranial injection142. The idea is that the spike protein persists in the plasma of PASC patients, not per se in the brain, and that (parts of) the protein causes alterations in multiple processes in the body, including neurological dysfunction. After infusion the level of neuroinflammation and memory dysfunction were increased142. Another approach is the use of the human lung-only mice, where immune deficient mice are implanted with human lung tissue and subsequently exposed to SARS-CoV-2. Infection-associated damage was distributed over the entire lung, however, no signs of cell loss were detected in the surrounding tissues in the follow-up period of maximum 14 days143. This makes this model solely relevant for studying certain aspects in the lungs but not in the rest of the body.
Discussion
In order to assess the potential of animal models for studying PASC, it is essential to evaluate what has been learned so far from animal models regarding SARS-CoV-2 infection and the origin of PASC. The value of experimental animal models lies in studying the pathophysiology of PASC and addressing knowledge gaps, but also in enabling access to ex vivo material, which is often difficult to obtain from patients. Furthermore, experimental models are commonly required during the initial stages of testing novel therapeutics.
To date, all studies indicate that the main hypotheses for the induction of PASC, as outlined in the introduction, remain valid, as no definitive answers have yet been found. Animal studies further support the plausibility of these hypotheses. However, the studies in animal models indicate that no single hypothesis fully explains the development of PASC; rather, the proposed mechanisms appear to be interconnected and correlated.
What makes it challenging to translate findings from animals to the clinical situation is that many animal models do not exhibit clinical symptoms, and alterations such as fatigue can only be measured indirectly, similar to humans. In general, it is in the character of animals to show signs of disease as little as possible. This aligns with the observation that despite the absence of obvious clinical symptoms, pathology induced by a SARS-CoV-2 infection can still be detected47,144. These measurements are typically obtained via blood sampling, cerebrospinal fluid (CSF) sampling, BALs, imaging techniques, and behavioral testing. These insights underscore the importance of translational readouts for visualizing and understanding the long-term consequences of a SARS-CoV-2 infection in vivo, and they support the need for longitudinal studies in both clinical and experimental models to investigate PASC.
As previously postulated7, regardless of the infection route or animal model used, the acute phase of a SARS-CoV-2 infection leads to a disturbance of the homeostasis. This disruption, either directly or indirectly, results in pathology combined with immune cell involvement, which can persist over time. Neural networks are particularly sensitive to such homeostatic disturbances and share essential information between the lung and the brain, two of the most prominent affected organs in both the active phase of the infection and PASC. Studying the consequences of infection in the most representative manner requires an intact and fully functional system. Therefore, the manipulation of animal models by implants of human lung tissue, feces, or the infusion of the spike protein will provide us with organ-specific information they do not allow for a comprehensive understanding of systemic effects. In this context, imaging studies using PASC animal models should shift to a multiple-system or total-body approach to allow for assessment of the complete system.
In addition to the currently recognized phases of PASC animal models also offer the potential to reveal long-term consequences, for instance through ex vivo analysis of organs, suggesting possible long-term health risks, such as an increased risk for neurodegenerative diseases145,146, like PD88,147 and AD148 as well as alterations in physiology of the heart that may lead to elevated cardiovascular disease risk149,150. The mechanisms underlying these pathologies are currently unclear and are beyond the scope of this review. However, to elucidate these mechansisms, it is essential that animal models faithfully replicate similar (neuro)pathological features associated with PASC.
Conclusion
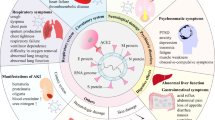
In humans, a SARS-CoV-2 infection, its sequelae, and potential development of PASC are heterogeneous. This heterogeneity is also observed in experimental animal models. While several models are well-suited for specific research questions, no single model can fully replicate all aspects of PASC (Fig. 1). This review provides an overview of the current status of animal models for PASC and aims to support researchers in selecting the most appropriate model for their specific research question.
PASC-related research applications include the immune system, blood circulation, central nervous system (CNS), metabolism, and organ and bone, cartilage, and muscle (BCM) tissue. Pie charts show the ratio of mice (purple), Syrian golden hamsters (orange), and NHPs (blue) used in studies for each research subject.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- ACE2:
-
Angiotensin-converting enzyme 2
- AD:
-
Alzheimer’s disease
- AGM:
-
African Green monkey
- APP:
-
Amyloid precursor protein
- AVV:
-
Adeno-associated virus
- BAL:
-
Bronchoalveolar lavage
- BCM:
-
Bone muscle cartilage
- BCS:
-
Body condition score (including body temperature, weight, and viral load)
- BPRC:
-
Biomedical Primate Research Centre
- CM:
-
Cynomolgus macaque
- CNS:
-
Central nervous system
- CoM:
-
Common marmoset
- COVID-19:
-
Corona disease 2029
- CSF:
-
Cerebrospinal fluid
- (μ)CT:
-
(micro) Computed tomography
- CTI:
-
Centre for Translational Immunology
- CTL:
-
Cytotoxic T lymphocyte
- d:
-
Days
- dpi:
-
Days post-infection
- ECG:
-
Electrocardiogram
- FDG:
-
Fluorodeoxyglucose
- GFAP:
-
Glial fibrillary acidic protein
- hACE2:
-
Human angiotensin converting enzyme 2
- HE:
-
Haematoxylin & eosin
- HFD:
-
High fat diet
- HLA:
-
Human leukocyte antigen
- hPFF:
-
Human preformed fibrils
- hTMPRSS2:
-
Human transmembrane serine protease 2
- H1N1:
-
Hemagglutin 1 neuraminidase 1
- Ig:
-
Immunoglobulin
- IFN:
-
Interferon
- IL:
-
Interleukin
- LNP:
-
Lipid nanoparticle
- LVV:
-
Lentiviral vector
- m:
-
Months
- ME/CFS:
-
Myalgic encephalomyelitis/chronic fatigue syndrome
- MHV(-1):
-
Murine hepatitis virus (strain 1)
- MPTP:
-
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- MRI:
-
Magnetic resonance imaging
- MVA:
-
Modified vaccinia Ankara
- NK:
-
Natural killer
- m + f:
-
Male female
- NASH:
-
Nonalcoholic steatohepatitis
- NCF:
-
Normal chow fed
- NETs:
-
Neutrophil extracellular traps
- NHPs:
-
Non-human primates
- PASC:
-
Post-acute sequelae of COVID-19
- PCDH:
-
Protocadherin
- PD:
-
Parkinson’s disease
- PEM:
-
Post-exertional malaise
- PET:
-
Positron emission tomography
- PET-CT:
-
Positron emission tomography-computed tomography
- Pi:
-
Post-infection
- RAS:
-
Renin-angiotensin system
- RBD:
-
Receptor-binding domain
- PBMC:
-
Peripheral blood mononuclear cell
- RM:
-
Rhesus macaque
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus 2
- SHIRPA:
-
SmithKline Beecham, Harwell, Imperial College, Royal London Hospital, Phenotype Assessment
- TSPO:
-
Translocator protein
- VOC:
-
Variant of concern
- w:
-
Weeks
- WHO:
-
World Health Organization
- y:
-
Years
References
WHO. WHO chief declares end to COVID-19 as a global health emergency. https://news.un.org/en/story/2023/05/1136367 (2023).
Al-Aly, Z. et al. Long COVID science, research and policy. Nat. Med. 30, 2148–2164 (2024).
Brus, I. M. et al. The prolonged impact of COVID-19 on symptoms, health-related quality of life, fatigue and mental well-being: a cross-sectional study. Front. Epidemiol. 3, 1144707 (2023).
L, O. M. et al. Impact of Long COVID on health and quality of life. HRB Open Res. 5, 31 (2022).
WHO. WHO COVID-19 dashboard. 2024. [cited 2024 19th of November]; Available from: https://data.who.int/dashboards/covid19/cases?n=c.
Davis, H. E. et al. Long COVID: major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 21, 133–146 (2023).
Schafer, A. et al. Animal models of Long Covid: a hit-and-run disease. Sci. Transl. Med. 16, eado2104 (2024).
Ye, G. et al. The long COVID symptoms and severity score: development, validation, and application. Value Health 27, 1085–1091 (2024).
Davis, H. E. et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine 38, 101019 (2021).
Proal, A. D. & VanElzakker, M. B. Long COVID or post-acute sequelae of COVID-19 (PASC): an overview of biological factors that may contribute to persistent symptoms. Front. Microbiol. 12, 698169 (2021).
Jansen, E. B. et al. After the virus has cleared-Can preclinical models be employed for Long COVID research?. PLoS Pathog. 18, e1010741 (2022).
Usai, C. et al. Animal models to study the neurological manifestations of the post-COVID-19 condition. Lab Anim.52, 202–210 (2023).
Chu, H., Chan, J. F. & Yuen, K. Y. Animal models in SARS-CoV-2 research. Nat. Methods 19, 392–394 (2022).
Casel, M. A. B., Rollon, R. G. & Choi, Y. K. Experimental animal models of coronavirus infections: strengths and limitations. Immune Netw. 21, e12 (2021).
Cleary, S. J. et al. Animal models of mechanisms of SARS-CoV-2 infection and COVID-19 pathology. Br. J. Pharmacol. 177, 4851–4865 (2020).
Munoz-Fontela, C. et al. Animal models for COVID-19. Nature 586, 509–515 (2020).
Peluso, M. J. & Deeks, S. G. Mechanisms of long COVID and the path toward therapeutics. Cell 187, 5500–5529 (2024).
Killingley, B. et al. Safety, tolerability and viral kinetics during SARS-CoV-2 human challenge in young adults>. Nat. Med. 28, 1031–1041 (2022).
Trender, W. et al. Changes in memory and cognition during the SARS-CoV-2 human challenge study. EClinicalMedicine 76, 102842 (2024).
Cohen, A. A. Aging across the tree of life: the importance of a comparative perspective for the use of animal models in aging. Biochim. Biophys. Acta Mol. Basis Dis. 1864, 2680–2689 (2018).
Yuan, R. et al. Relationships among development, growth, body size, reproduction, aging, and longevity - trade-offs and pace-of-life. Biochem. (Mosc. 88, 1692–1703 (2023).
Xie, Y., Choi, T. & Al-Aly, Z. Postacute sequelae of SARS-CoV-2 infection in the pre-delta, delta, and omicron eras. N. Engl. J. Med. 391, 515–525 (2024).
de Melo, G. D. et al. Neuroinvasion and anosmia are independent phenomena upon infection with SARS-CoV-2 and its variants. Nat. Commun. 14, 4485 (2023).
Fumagalli, V. et al. Administration of aerosolized SARS-CoV-2 to K18-hACE2 mice uncouples respiratory infection from fatal neuroinvasion. Sci. Immunol. 7, eabl9929 (2022).
Jeon, D. et al. Discovery of a new long COVID mouse model via systemic histopathological comparison of SARS-CoV-2 intranasal and inhalation infection. Biochim. Biophys. Acta Mol. Basis Dis. 1870, 167347 (2024).
Stammes, M. A. et al. Medical imaging of pulmonary disease in SARS-CoV-2-exposed non-human primates. Trends Mol. Med. 28, 123–142 (2022).
Saravanan, U. B. et al. Animal models for SARS-CoV-2 and SARS-CoV-1 pathogenesis, transmission and therapeutic evaluation. World J. Virol. 11, 40–56 (2022).
Dong, W. et al. The K18-human ACE2 transgenic mouse model recapitulates non-severe and severe COVID-19 in response to an infectious dose of the SARS-CoV-2 virus. J. Virol. 96, e0096421 (2022).
Izadpanah, A. et al. SARS-CoV-2 infection dysregulates NAD metabolism. Front. Immunol. 14, 1158455 (2023).
Liu, H. et al. Establishment and characterization of an hACE2/hTMPRSS2 knock-in mouse model to study SARS-CoV-2. Front. Immunol. 15, 1428711 (2024).
Hoffmann, M. et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181, 271–280 e8 (2020).
Puntoni, M. et al. Impact of age and sex interaction on post-acute sequelae of COVID-19: an italian cohort study on adults and children. J. Clin. Med. 12, (2023).
Dumont, R. et al. A population-based serological study of post-COVID syndrome prevalence and risk factors in children and adolescents. Nat. Commun. 13, 7086 (2022).
Stephenson, T. et al. Physical and mental health 3 months after SARS-CoV-2 infection (long COVID) among adolescents in England (CLoCk): a national matched cohort study. Lancet Child Adolesc. Health 6, 230–239 (2022).
Lelii, F. & Leeson, R. COVID-19 Schools Infection Survey, England: pupil antibody data and vaccine sentiment, March to April 2022. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/covid19schoolsinfectionsurveyengland/pupilantibodiesandvaccinesentimentmarch2022 (2022).
Bai, F. et al. Female gender is associated with long COVID syndrome: a prospective cohort study. Clin. Microbiol. Infect. 28, 611 e9–611 e16 (2022).
Bucciarelli, V. et al. Depression pandemic and cardiovascular risk in the COVID-19 era and long COVID syndrome: gender makes a difference. Trends Cardiovasc. Med. 32, 12–17 (2022).
Pilchova, V. et al. Characterization of young and aged ferrets as animal models for SARS-CoV-2 infection with focus on neutrophil extracellular traps. Front. Immunol. 14, 1283595 (2023).
Francis, M. E. et al. Sex and age bias viral burden and interferon responses during SARS-CoV-2 infection in ferrets. Sci. Rep. 11, 14536 (2021).
Speranza, E. et al. Age-related differences in immune dynamics during SARS-CoV-2 infection in rhesus macaques. Life Sci. Alliance 5, e202101314 (2022).
Rockx, B. et al. Comparative pathogenesis of COVID-19, MERS, and SARS in a nonhuman primate model. Science 368, 1012–1015 (2020).
Rouhana, S. et al. Sex differences in the cardiac stress response following SARS-CoV-2 infection of ferrets. Am. J. Physiol. Heart Circ. Physiol. 325, H1153–H1167 (2023).
Coleon, A. Hamsters with long COVID exhibits a neurodegenerative signature in the brainstem. bioRxiv https://doi.org/10.1101/2024.12.16.628627 (2024).
Facciuolo, A. et al. Longitudinal analysis of SARS-CoV-2 reinfection reveals distinct kinetics and emergence of cross-neutralizing antibodies to variants of concern. Front. Microbiol. 14, 1148255 (2023).
Mohandas, S. et al. Protective immunity of the primary SARS-CoV-2 infection reduces disease severity post re-infection with delta variants in Syrian hamsters. Viruses 14, 596 (2022).
Field, C. J. et al. Immune durability and protection against SARS-CoV-2 re-infection in Syrian hamsters. Emerg. Microbes Infect. 11, 1103–1114 (2022).
Nieuwland, J. M. et al. Longitudinal positron emission tomography and postmortem analysis reveals widespread neuroinflammation in SARS-CoV-2 infected rhesus macaques. J. Neuroinflam. 20, 179 (2023).
Meijer, L. et al. Novel application of [(18)F]DPA714 for visualizing the pulmonary inflammation process of SARS-CoV-2-infection in rhesus monkeys (Macaca mulatta). Nucl. Med. Biol. 112-113, 1–8 (2022).
Palard-Novello, X.V. et al. Whole-body [18F]DPA-714 kinetic assessment using PET/CT scanner with long axial field of view. J. Nucl. Med. 66, 1142–1148 (2025).
Mooij, P. et al. Poxvirus MVA expressing SARS-CoV-2 S protein induces robust immunity and protects rhesus macaques from SARS-CoV-2. Front. Immunol. 13, 845887 (2022).
Lindeboom, R. G. H. et al. Human SARS-CoV-2 challenge uncovers local and systemic response dynamics. Nature 631, 189–198 (2024).
Cheong, J. G. et al. Epigenetic memory of coronavirus infection in innate immune cells and their progenitors. Cell 186, 3882–3902 e24 (2023).
Salguero, F. J. et al. Comparison of rhesus and cynomolgus macaques as an infection model for COVID-19. Nat. Commun. 12, 1260 (2021).
Maaskant, A. et al. Bronchoalveolar lavage affects thorax computed tomography of healthy and SARS-CoV-2 infected rhesus macaques (Macaca mulatta). PLoS ONE 16, e0252941 (2021).
Huot, N. et al. SARS-CoV-2 viral persistence in lung alveolar macrophages is controlled by IFN-gamma and NK cells. Nat. Immunol. 24, 2068–2079 (2023).
Nelson, C. E. et al. IL-10 suppresses T cell expansion while promoting tissue-resident memory cell formation during SARS-CoV-2 infection in rhesus macaques. PLoS Pathog. 20, e1012339 (2024).
Melton, A. et al. The pigtail macaque (Macaca nemestrina) model of COVID-19 reproduces diverse clinical outcomes and reveals new and complex signatures of disease. PLoS Pathog. 17, e1010162 (2021).
Chiuppesi, F. et al. Synthetic multiantigen MVA vaccine COH04S1 protects against SARS-CoV-2 in Syrian hamsters and non-human primates. NPJ Vaccines 7, 7 (2022).
Giannakopoulos, S. et al. Post-COVID pulmonary injury in K18-hACE2 mice shows persistent neutrophils and neutrophil extracellular trap formation. Immun. Inflamm. Dis. 12, e1343 (2024).
Hsu, C. J. et al. Dynamic changes of the blood chemistry in syrian hamsters post-acute COVID-19. Microbiol. Spectr. 10, e0236221 (2022).
Becker, K. et al. Vasculitis and neutrophil extracellular traps in lungs of golden syrian hamsters with SARS-CoV-2. Front Immunol. 12, 640842 (2021).
Vilas Boas de Melo, C. et al. Influenza Infection in Ferrets with SARS-CoV-2 Infection History. Microbiol. Spectr. 10, e0138622 (2022).
Kreft, I. C. et al. Absence of COVID-19-associated changes in plasma coagulation proteins and pulmonary thrombosis in the ferret model. Thromb. Res. 210, 6–11 (2022).
Mina, Y. et al. Deep phenotyping of neurologic postacute sequelae of SARS-CoV-2 infection. Neurol. Neuroimmunol. Neuroinflamm. 10, e200097 (2023).
Gagliardi, S. et al. Detection of SARS-CoV-2 genome and whole transcriptome sequencing in frontal cortex of COVID-19 patients. Brain Behav. Immun. 97, 13–21 (2021).
Meinhardt, J. et al. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat. Neurosci. 24, 168–175 (2021).
Thakur, K. T. et al. COVID-19 neuropathology at Columbia University Irving Medical Center/New York Presbyterian Hospital. Brain 144, 2696–2708 (2021).
Schwabenland, M. et al. Deep spatial profiling of human COVID-19 brains reveals neuroinflammation with distinct microanatomical microglia-T-cell interactions. Immunity 54, 1594–1610 e11 (2021).
Nersesjan, V. et al. SARS-CoV-2 and autoantibodies in the cerebrospinal fluid of COVID-19 patients: prospective multicentre cohort study. Brain Commun. 5, fcad274 (2023).
Rutkai, I. et al. Neuropathology and virus in brain of SARS-CoV-2 infected non-human primates. Nat. Commun. 13, 1745 (2022).
Golden, J.W. et al. Human convalescent plasma protects K18-hACE2 mice against severe respiratory disease. J. Gen. Virol. 102, 001599 (2021).
Li, H. et al. SARS-CoV-2 RNA persists in the central nervous system of non-human primates despite clinical recovery. Mol. Biomed. 4, 39 (2023).
Monchatre-Leroy, E. et al. Hamster and ferret experimental infection with intranasal low dose of a single strain of SARS-CoV-2. J. Gen. Virol. 102, 001567 (2021).
Jiao, L. et al. The olfactory route is a potential way for SARS-CoV-2 to invade the central nervous system of rhesus monkeys. Signal. Transduct. Target Ther. 6, 169 (2021).
Beckman, D. et al. SARS-CoV-2 infects neurons and induces neuroinflammation in a non-human primate model of COVID-19. Cell Rep. 41, 111573 (2022).
Poole-Wright, K. et al. Fatigue outcomes following COVID-19: a systematic review and meta-analysis. BMJ Open 13, e063969 (2023).
Yan, K. et al. Establishment and identification of an animal model of long-term exercise-induced fatigue. Front Endocrinol. 13, 915937 (2022).
Stutz, P. V., Golani, L. K. & Witkin, J. M. Animal models of fatigue in major depressive disorder. Physiol. Behav. 199, 300–305 (2019).
Chen, J. et al. Amyloid precursor protein facilitates SARS-CoV-2 virus entry into cells and enhances amyloid-beta-associated pathology in APP/PS1 mouse model of Alzheimer’s disease. Transl. Psychiatry 13, 396 (2023).
Smeyne, R. J. et al. COVID-19 Infection Enhances Susceptibility to Oxidative Stress-Induced Parkinsonism. Mov. Disord. 37, 1394–1404 (2022).
Kishimoto-Urata, M. et al. Prolonged and extended impacts of SARS-CoV-2 on the olfactory neurocircuit. Sci. Rep. 12, 5728 (2022).
Schreiber, C. S. et al. Sex-specific biphasic alpha-synuclein response and alterations of interneurons in a COVID-19 hamster model. EBioMedicine 105, 105191 (2024).
Vidal, E. et al. Chronological brain lesions after SARS-CoV-2 infection in hACE2-transgenic mice. Vet. Pathol. 59, 613–626 (2022).
Wood, G. K. et al. Posthospitalization COVID-19 cognitive deficits at 1 year are global and associated with elevated brain injury markers and gray matter volume reduction. Nat. Med. 31, 245–257 (2024).
VanElzakker, M. B. et al. Neuroinflammation in post-acute sequelae of COVID-19 (PASC) as assessed by [(11)C]PBR28 PET correlates with vascular disease measures. Brain Behav. Immun. 119, 713–723 (2024).
Reiken, S. et al. Alzheimer ’s-like signaling in brains of COVID-19 patients. Alzheimers Dement 18, 955–965 (2022).
Brundin, P., Nath, A. & Beckham, J. D. Is COVID-19 a perfect storm for Parkinson’s disease?. Trends Neurosci. 43, 931–933 (2020).
Philippens, I. et al. Brain Inflammation and Intracellular alpha-Synuclein Aggregates in Macaques after SARS-CoV-2 infection. Viruses 14, 776 (2022).
Claessens, G. et al. Prevalence and predictors of persistent cognitive and psychological symptoms in non-hospitalized post-COVID-19 patients seeking care at an outpatient post-COVID-19 clinic. Front. Psychol. 15, 1396963 (2024).
Singh, A. et al. A murine model of post-acute neurological sequelae following SARS-CoV-2 variant infection. Front. Immunol. 15, 1384516 (2024).
Frere, J. J. et al. SARS-CoV-2 infection in hamsters and humans results in lasting and unique systemic perturbations after recovery. Sci. Transl. Med. 14, eabq3059 (2022).
Saito, S. et al. Metabolomic and immune alterations in long COVID patients with chronic fatigue syndrome. Front. Immunol. 15, 1341843 (2024).
Lage, S. L. et al. Persistent immune dysregulation and metabolic alterations following SARS-CoV-2 infection. medRxiv https://doi.org/10.1101/2025.04.16.25325949 (2025).
Briand, F. et al. Diet-induced obesity and NASH impair disease recovery in SARS-CoV-2-infected golden hamsters. Viruses 14, 2067 (2022).
Cao, X. et al. The SARS-CoV-2 spike protein induces long-term transcriptional perturbations of mitochondrial metabolic genes, causes cardiac fibrosis, and reduces myocardial contractile in obese mice. Mol. Metab. 74, 101756 (2023).
Sokol, H. et al. SARS-CoV-2 infection in nonhuman primates alters the composition and functional activity of the gut microbiota. Gut Microbes 13, 1–19 (2021).
Palmer, C. S. et al. Non-human primate model of long-COVID identifies immune associates of hyperglycemia. Nat. Commun. 15, 6664 (2024).
Michaels, T.M., Essop, M.F. & Joseph, D.E. Potential effects of hyperglycemia on SARS-CoV-2 entry mechanisms in pancreatic beta cells. Viruses 16,1243 (2024).
Dinnon, K. H. 3rd et al. SARS-CoV-2 infection produces chronic pulmonary epithelial and immune cell dysfunction with fibrosis in mice. Sci. Transl. Med. 14, eabo5070 (2022).
Li, C. et al. Chronic lung inflammation and CK14+ basal cell proliferation induce persistent alveolar-bronchiolization in SARS-CoV-2-infected hamsters. EBioMedicine 108, 105363 (2024).
Ramamoorthy, R. et al. Kidney damage in long COVID: studies in experimental mice. Biology 12, 1070 (2023).
Rizvi, Z.A. et al. Golden Syrian hamster as a model to study cardiovascular complications associated with SARS-CoV-2 infection. Elife 11, e73522 (2022).
Qiao, W. et al. SARS-CoV-2 infection induces inflammatory bone loss in golden Syrian hamsters. Nat. Commun. 13, 2539 (2022).
Appelman, B. et al. Muscle abnormalities worsen after post-exertional malaise in long COVID. Nat. Commun. 15, 17 (2024).
Soares, M. N. et al. Skeletal muscle alterations in patients with acute Covid-19 and post-acute sequelae of Covid-19. J. Cachexia Sarcopenia Muscle 13, 11–22 (2022).
Au, M. T. et al. Blockade of endothelin receptors mitigates SARS-CoV-2-induced osteoarthritis. Nat. Microbiol. 9, 2538–2552 (2024).
Kocyigit, B. F. & Akyol, A. Reactive arthritis after COVID-19: a case-based review. Rheumatol. Int. 41, 2031–2039 (2021).
Gagiannis, D. et al. Clinical, imaging, and histopathological features of pulmonary sequelae after mild COVID-19. Am. J. Respir. Crit. Care Med. 208, 618–621 (2023).
Solomon, J. J. et al. CT of post-acute lung complications of COVID-19. Radiology 301, E383–E395 (2021).
Comellas, A.P. & Fain, S.B. Lung MRI identifies potentially treatable subtypes of long COVID. Eur. Respir. J. 63, 2400381 (2024).
Deng, W. et al. Primary exposure to SARS-CoV-2 protects against reinfection in rhesus macaques. Science 369, 818–823 (2020).
Boszormenyi, K.P. et al. The post-acute phase of SARS-CoV-2 infection in two macaque species is associated with signs of ongoing virus replication and pathology in pulmonary and extrapulmonary tissues. Viruses 2021. 13.
Hartman, A. L. et al. SARS-CoV-2 infection of African green monkeys results in mild respiratory disease discernible by PET/CT imaging and shedding of infectious virus from both respiratory and gastrointestinal tracts. PLoS Pathog. 16, e1008903 (2020).
Adney, D.R. et al. Severe acute respiratory disease in American mink experimentally infected with SARS-CoV-2. JCI Insight 7, 1673 (2022).
Blair, R. V. et al. Acute respiratory distress in aged, SARS-CoV-2-infected african green monkeys but not rhesus macaques. Am. J. Pathol. 191, 274–282 (2021).
Harris, P.E. et al. A synthetic peptide CTL vaccine targeting nucleocapsid confers protection from SARS-CoV-2 challenge in rhesus macaques. Vaccines 9, 520 (2021).
Johnston, S. C. et al. Development of a coronavirus disease 2019 nonhuman primate model using airborne exposure. PLoS ONE 16, e0246366 (2021).
Munster, V. J. et al. Respiratory disease in rhesus macaques inoculated with SARS-CoV-2. Nature 585, 268–272 (2020).
Lu, S. et al. Comparison of nonhuman primates identified the suitable model for COVID-19. Signal. Transduct. Target Ther. 5, 157 (2020).
Pillet, S. et al. Safety, immunogenicity, and protection provided by unadjuvanted and adjuvanted formulations of a recombinant plant-derived virus-like particle vaccine candidate for COVID-19 in nonhuman primates. Cell Mol. Immunol. 19, 222–233 (2022).
Yu, P. et al. Age-related rhesus macaque models of COVID-19. Anim. Model Exp. Med. 3, 93–97 (2020).
Castro, M. A. et al. Toward the determination of sensitive and reliable whole-lung computed tomography features for robust standard radiomics and delta-radiomics analysis in a nonhuman primate model of coronavirus disease 2019. J. Med. Imaging 9, 066003 (2022).
Reza, S. M. S. et al. Deep-learning-based whole-lung and lung-lesion quantification despite inconsistent ground truth: application to computerized tomography in SARS-CoV-2 nonhuman primate models. Acad. Radio. 30, 2037–2045 (2023).
Stammes, M.A. Non-invasive monitoring of inflammatory processes by myeloid cell-directed PET tracers in an experimental SARS-CoV-2 infection model. J. Nucl. Med.13, 2124–2137 (2025).
Koopman, G. et al. Imaging the immune sequelae of infection with SARS-CoV-2 in nonhuman primates by using two nanobody PET-tracers. J. Med. Virol. 96, e29956 (2024).
Lemaitre, J. et al. Non-human primate models of human respiratory infections. Mol. Immunol. 135, 147–164 (2021).
Detrille, A. et al. Whole-body visualization of SARS-CoV-2 biodistribution in vivo by immunoPET imaging in non-human primates. Nat. Commun. 16, 2816 (2025).
Boszormenyi, K. P. et al. Prolonged fecal shedding of replication-competent virus, lasting immune activation, and intestinal inflammation in a rhesus macaque after experimental SARS-CoV-2 infection. Front. Cell Infect. Microbiol. 14, 1505720 (2024).
Finch, C.L. et al. Characteristic and quantifiable COVID-19-like abnormalities in CT- and PET/CT-imaged lungs of SARS-CoV-2-infected crab-eating macaques (Macaca fascicularis). bioRxiv https://doi.org/10.1101/2020.05.14.096727 (2020).
Cull, O. et al. Radiological markers of neurological manifestations of post-acute sequelae of SARS-CoV-2 infection: a mini-review. Front. Neurol. 14, 1233079 (2023).
Kang, D., Jung, H. & Pak, K. Altered brain glucose metabolism in COVID-19 disease: an activation likelihood estimation meta-analysis of PET studies. Brain Imaging Behav. 19, 313–322 (2025).
Verger, A.B., et al, E., 2-[18F]-FDG PET for imaging brain involvement in patients with long COVID: perspective of the EANM Neuroimaging Committee. Eur. J. Nucl. Med. Mol. Imaging 49, 3599–3606 (2022).
Douaud, G. et al. SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature 604, 697–707 (2022).
Lu, J. et al. Cerebral small vessel injury in mice with damage to ACE2-expressing cerebral vascular endothelial cells and post COVID-19 patients. Alzheimers Dement 20, 7971–7988 (2024).
Stapleton-Kotloski, J. R. et al. Magnetoencephalography reveals neuroprotective effects of COVID-19 vaccination in non-human primates. bioRxiv, https://doi.org/10.1101/2025.02.14.638187 (2025).
Cao, X. et al. The SARS-CoV-2 Spike protein induces long-term transcriptional perturbations of mitochondrial metabolic genes, causes cardiac fibrosis, and reduces myocardial contractile in obese mice. bioRxiv, https://doi.org/10.1101/2025.02.14.638187 (2023).
Caldera-Crespo, L. A. et al. Experimental models of COVID-19. Front. Cell Infect. Microbiol. 11, 792584 (2021).
Shou, S. et al. Animal models for COVID-19: hamsters, mouse, ferret, mink, tree shrew, and non-human primates. Front. Microbiol. 12, 626553 (2021).
Mendes de Almeida, V. et al. Gut microbiota from patients with COVID-19 cause alterations in mice that resemble post-COVID symptoms. Gut Microbes 15, 2249146 (2023).
Chen, H. Transfer of IgG from Long COVID patients induces symptomology in mice. bioRxiv https://doi.org/10.1101/2024.05.30.596590 (2024).
Santos Guedes de Sa, K. A causal link between autoantibodies and neurological symptoms in long COVID. medRxiv, https://doi.org/10.1101/2024.06.18.24309100 (2024).
Fontes-Dantas, F. L. et al. SARS-CoV-2 Spike protein induces TLR4-mediated long-term cognitive dysfunction recapitulating post-COVID-19 syndrome in mice. Cell Rep. 42, 112189 (2023).
Wahl, A. et al. SARS-CoV-2 infection is effectively treated and prevented by EIDD-2801. Nature 591, 451–457 (2021).
van de Ven, K. et al. Pathology and immunity after SARS-CoV-2 infection in male ferrets is affected by age and inoculation route. Front. Immunol. 12, 750229 (2021).
Bonhenry, D. et al. SARS-CoV-2 infection as a cause of neurodegeneration. Lancet Neurol. 23, 562–563 (2024).
Iacono, S. et al. COVID-19 and neurological disorders: what might connect Parkinson’s disease to SARS-CoV-2 infection. Front. Neurol. 14, 1172416 (2023).
Mysiris, D. S. et al. Post-COVID-19 Parkinsonism and Parkinson’s disease pathogenesis: the exosomal cargo hypothesis. Int. J. Mol. Sci. 23, 9739 (2022).
Shan, D. et al. Association between COVID-19 infection and new-onset dementia in older adults: a systematic review and meta-analysis. BMC Geriatr. 24, 940 (2024).
Blasi, F., M. Vicenzi, & De Ponti, R. COVID-19 and cardiac arrhythmias: lesson learned and dilemmas. J. Clin. Med. 13. 2024.
Marwick, T.H. et al. Cardiac function and functional capacity in patients with long COVID: a comparison to propensity-matched community controls. J. Am. Soc. Echocardiogr. 38, 16–23.e1 (2024).
Masciarella, A. D. et al. A mouse model of MHV-1 virus infection for study of acute and long COVID infection. Curr. Protoc. 3, e896 (2023).
Hussain, H. et al. Acute and long COVID intestinal changes in an experimental model of coronavirus in mice. Viruses 16, 832 (2024).
Paidas, M. J. et al. Long-term sequelae of COVID-19 in experimental mice. Mol. Neurobiol. 59, 5970–5986 (2022).
Verma, A.K. et al. Persistent neurological deficits in mouse PASC reveal antiviral drug limitations. bioRxiv, https://doi.org/10.1101/2024.06.02.596989 (2024).
Cui, L. et al. Innate immune cell activation causes lung fibrosis in a humanized model of long COVID. Proc. Natl. Acad. Sci. USA 120, e2217199120 (2023).
Gressett, T.E. et al. Mouse adapted SARS-CoV-2 model induces “long-COVID” neuropathology in BALB/c Mice. bioRxiv, https://doi.org/10.1101/2023.03.18.533204 (2023).
Fernandez-Castaneda, A. et al. Mild respiratory SARS-CoV-2 infection can cause multi-lineage cellular dysregulation and myelin loss in the brain. bioRxiv, https://doi.org/10.1016/j.cell.2022.06.008 (2022).
Lee, B. et al. SARS-CoV-2 infection exacerbates the cellular pathology of Parkinson’s disease in human dopaminergic neurons and a mouse model. Cell Rep. Med. 5, 101570 (2024).
Ma, T. et al. Post-acute immunological and behavioral sequelae in mice after Omicron infection. bioRxiv, https://doi.org/10.1101/2023.06.05.543758 (2023).
Somogyi, E. et al. T cell immunity ameliorates COVID-19 disease severity and provides post-exposure prophylaxis after peptide-vaccination, in Syrian hamsters. Front. Immunol. 14, 1111629 (2023).
Reyna, R. A. et al. Recovery of anosmia in hamsters infected with SARS-CoV-2 is correlated with repair of the olfactory epithelium. Sci. Rep. 12, 628 (2022).
Li, C. et al. Comparative single-cell analysis reveals IFN-gamma as a driver of respiratory sequelae after acute COVID-19. Sci. Transl. Med. 16, eadn0136 (2024).
Ma, G. et al. SARS-CoV-2 Spike protein S2 subunit modulates gamma-secretase and enhances amyloid-beta production in COVID-19 neuropathy. Cell Discov. 8, 99 (2022).
Serafini, R. A. et al. SARS-CoV-2 airway infection results in the development of somatosensory abnormalities in a hamster model. Sci. Signal. 16, eade4984 (2023).
Berry, N. et al. Intrinsic host susceptibility among multiple species to intranasal SARS-CoV-2 identifies diverse virological, biodistribution and pathological outcomes. Sci. Rep. 12, 18694 (2022).
Bosco-Lauth, A. M. et al. Experimental infection of brazilian free-tailed bats (Tadarida brasiliensis) with two strains of SARS-CoV-2. Viruses 14,1809 (2022).
Choi, C. Y. et al. Generation and characterization of a humanized ACE2 mouse model to study long-term impacts of SARS-CoV-2 infection. J. Med. Virol. 96, e29349 (2024).
Yinda, C. K. et al. K18-hACE2 mice develop respiratory disease resembling severe COVID-19. PLoS Pathog. 17, e1009195 (2021).
Delval, L. et al. Removal of senescent cells reduces the viral load and attenuates pulmonary and systemic inflammation in SARS-CoV-2-infected, aged hamsters. Nat. Aging 3, 829–845 (2023).
Barroso-Arévalo, S. et al. Comparative SARS-CoV-2 Omicron BA.5 variant and D614G-Wuhan strain infections in ferrets: insights into attenuation and disease progression during subclinical to mild COVID-19. Front. Vet. Sci. 11, 1435464 (2024).
Everett, H.E. et al. Intranasal infection of ferrets with SARS-CoV-2 as a model for asymptomatic human infection. Viruses 13, 113 (2021).
Oh, T., Hong, J. J. & Park, J. H. Histopathological pulmonary lesions in rhesus (Macaca mulatta) and cynomolgus (Macaca fascicularis) macaques experimentally infected with wild-type severe acute respiratory syndrome coronavirus 2. J. Comp. Pathol. 208, 5–10 (2024).
Nomura, T. et al. Subacute SARS-CoV-2 replication can be controlled in the absence of CD8+ T cells in cynomolgus macaques. PLoS Pathog. 17, e1009668 (2021).
Zheng, H. et al. Virulence and pathogenesis of SARS-CoV-2 infection in rhesus macaques: a nonhuman primate model of COVID-19 progression. PLoS Pathog. 16, e1008949 (2020).
Hall, J. S. et al. Experimental Infection of mexican free-tailed bats (Tadarida brasiliensis) with SARS-CoV-2. mSphere 8, e0026322 (2023).
Yang, M. S. et al. Ultra- and micro-structural changes of respiratory tracts in SARS-CoV-2 infected Syrian hamsters. Vet. Res. 52, 121 (2021).
Kolloli, A. et al. A phosphodiesterase-4 inhibitor reduces lung inflammation and fibrosis in a hamster model of SARS-CoV-2 infection. Front. Immunol. 14, 1270414 (2023).
Deng, Y. Q. et al. Lipid nanoparticle-encapsulated mRNA antibody provides long-term protection against SARS-CoV-2 in mice and hamsters. Cell Res. 32, 375–382 (2022).
Francis, M. E. et al. SARS-CoV-2 infection in the Syrian hamster model causes inflammation as well as type I interferon dysregulation in both respiratory and non-respiratory tissues including the heart and kidney. PLoS Pathog. 17, e1009705 (2021).
Bessière, P. et al. Intranasal type I interferon treatment is beneficial only when administered before clinical signs onset in the SARS-CoV-2 hamster model. PLoS Pathog. 17, e1009427 (2021).
Johansen, M. D. et al. Animal and translational models of SARS-CoV-2 infection and COVID-19. Mucosal Immunol. 13, 877–891 (2020).
Pandey, K. et al. Animal models for SARS-CoV-2 research: a comprehensive literature review. Transbound. Emerg. Dis. 68, 1868–1885 (2021).
Herbert, C. et al. Relationship between acute SARS-CoV-2 viral clearance and long COVID-19 (Long COVID) symptoms: a cohort study. Clin. Infect. Dis. 80, 82–90 (2025).
Acknowledgements
This work was initiated and partly supported by the Post Covid National Network (PCNN) project number 11080012310002 of ZonMw, especially WP6 which focus is on unraveling the pathophysiology of PASC. We acknowledge the assistance, input an lively discussions on this topic with all the members with a specific focus on; Magdalena Lorenowicz, Marieke van der Schaaf, Odilia Cornet, Brent Appelman, Xandra Westerhuis en Karin Meijer. We would like to thank Francisca van Hassel for her assistance with figure editing.
Author information
Authors and Affiliations
Contributions
W.B., J.A.M.L., J.M., and M.A.S. contributed to the conceptualization of the manuscript; Jvd.B., J.M., and M.A.S. contributed to data curation; Jvd.B., W.B., J.A.M.L., J.M., and M.A.S. had unrestricted access to all data; Jvd.B. and M.A.S. contributed to visualization; Jvd.B., J.M., and M.A.S. accessed and verified the data; Jvd.B., A.C., H.L.D.M.W., G.Dd.M., J.M., and M.A.S. contributed to writing of the original draft; and all authors contributed to the review and editing process of the manuscript. All authors agreed to submit the manuscript, read, and approve the final draft and take full responsibility of its content, including the accuracy of the data and the fidelity of the work performed. The Authors declare no Competing Financial or Non-Financial Interests.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
van der Bie, J., Coleon, A., Visser, D. et al. Post Pandemic Problem, is there an animal model suitable to investigate PASC. npj Imaging 3, 41 (2025). https://doi.org/10.1038/s44303-025-00101-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s44303-025-00101-2