Abstract
Candidatus Profftella armatura (Betaproteobacteria) is an organelle-like defensive symbiont inhabiting the symbiotic organ of a devastating citrus pest, the Asian citrus psyllid Diaphorina citri. Previous two-dimensional electron microscopy hinted at unprecedented ultrastructures in Profftella, but their precise architecture and composition were unknown. Here, using serial block-face scanning electron microscopy, high-voltage electron tomography, and fluorescence in situ hybridization, we show that elongated Profftella cells (2.8–136 μm observed) contain multiple tubes (1–43 per cell) up to 45 μm long. These tubes, occupying ~6.3% of the cell volume, are composed of five or six fibers twisted into a right-handed helix with a consistent diameter of ~230 nm. Their stability under high vacuum suggests a mechanical support role in elongated Profftella. Close association with ribosomes implies a possible role in protein synthesis. Our findings provide insight into the structural adaptations of intracellular symbionts and may inform strategies for controlling citrus pests.
Similar content being viewed by others
Introduction
In contrast to eukaryotes, bacteria typically have simpler intracellular structures, lacking the elaborate intracellular membrane systems and organelles found in eukaryotes1. Certain ultrastructures, occasionally referred to as ‘bacterial organelles,’ such as chromatophores2, carboxysomes3, metabolosomes4, and magnetosomes5, are found in specific bacterial lineages1. However, in most bacteria, the easily observable intracellular structures are limited only to ribosomes and, in some cases, nucleoids6. In this context, we recently found an unprecedented ultrastructure with a seemingly tubular shape in a bacterial symbiont of a sap-sacking insect, the Asian citrus psyllid Diaphorina citri (Hemiptera: Sternorrhyncha: Psylloidea: Psyllidae)7.
D. citri is a notorious agricultural pest that transmits “Candidatus Liberibacter” spp. (Alphaproteobacteria: Rhizobiales, hereafter Liberibacter), the pathogens of the most destructive and incurable citrus disease, huanglongbing8,9. D. citri possesses a symbiotic organ called the bacteriome10,11,12, which harbors two transovarially transmitted obligate intracellular bacterial mutualists, “Candidatus Carsonella ruddii” (Gammaproteobacteria: Oceanospirillales, hereafter Carsonella)13,14,15 and “Candidatus Profftella armatura” (Betaproteobacteria: Burkholderiales, hereafter Profftella)14,16,17,18. Carsonella, the primary symbiont conserved in Psylloidea13,14,15,16,17,18,19,20,21,22,23,24,25,26,27, is a typical nutritional symbiont providing the host with essential amino acids14,15,17,24,26,28 scarce in the phloem sap diet29,30. On the other hand, Profftella, a secondary symbiont found exclusively in Diaphorina spp14,16,17., is a unique, organelle-like, versatile symbiont whose primary role appears to be protecting the holobiont (host-symbiont complex) from natural enemies by synthesizing the bioactive polyketide diaphorin14,17,31,32,33,34. The Profftella genome is drastically reduced to 460 kb, with 15% dedicated to gene clusters for synthesizing diaphorin14. Diaphorin is present in D. citri at concentrations as high as 2–20 mM, depending on its developmental stage33. It is inhibitory to eukaryotes14,31,32 and various bacteria, including Bacillus subtilis (Firmicutes: Bacilli: Bacillales), but promotes the growth of a limited number of bacterial species, including Escherichia coli (Gammaproteobacteria: Enterobacterales)34,35. Cell-free gene expression analyses suggest that the ribosome is a target for both the inhibitory and promoting effects of diaphorin on bacteria36,37. This unique ability of diaphorin to modulate bacterial vital activities may influence the microbiota of D. citri and potentially affect the transmission of Liberibacter pathogens34. Similar to other hemipteran insects38,39,40,41,42,43,44,45,46,47,48,49, there is growing evidence that interactions among the host psyllid, bacteriome-associated obligate mutualists, facultative symbionts, and plant pathogens play critical roles in psyllid biology and host plant pathology9,11,18,50,51,52,53.
Despite its importance for understanding Profftella’s unique functions, evolution, and interactions with the host and other microbes, Profftella’s ultrastructures remain largely uncharacterized. Although our two-dimensional (2D) transmission electron microscopy (TEM) analysis showed that Profftella cells contain a seemingly tubular ultrastructure unprecedented in other bacteria7, its detailed architecture, three-dimensional (3D) arrangement within the host cell, and components remained unknown. To address these questions, in the present study, we first examined the fine 3D ultrastructure of the tube using serial block-face scanning electron microscopy (SBF-SEM) and high voltage electron microscopy (HVEM) tomography. SBF-SEM is a 3D imaging technique that generates 3D images by stacking serial section images, enabling high-resolution 3D visualization of the internal structures of biological samples54. Electron tomography, utilizing 1000 kV HVEM, enables high-resolution 3D observation of biological specimens as thick as 1 μm, owing to the high penetrating power of the electron beam55. As the putative spiral structure of the tube was somewhat similar to that of the nucleoid previously reported in Bdellovibrio bacteriovorus (Bdellovibrionota: Bdellovibrionia: Bdellovibrionales)56 and Synechococcus elongatus (Cyanobacteriota: Cyanophyceae: Synechococcales)57, we further analyzed the localization of DNA and RNA by performing DNA staining along with fluorescence in situ hybridization (FISH) using primers targeting the 16S ribosomal RNA (rRNA) of the bacteriome associates.
Results
Observation of Profftella and its tubes by optical microscopy and 2D TEM
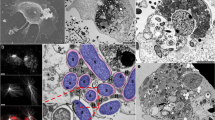
To obtain an overview of Profftella, cells of Profftella, which are tightly packed in the syncytium of the host bacteriome, were released from the dissected bacteriome and directly observed without fixation, using differential interference contrast (DIC) microscopy (Fig. 1A). The cells were large and elongated, exhibiting various lengths and apparent widths of 2–5 µm. The tubular structures previously inferred from our 2D TEM analysis were confirmed to exist through optical microscopy, revealing that each Profftella cell contains numerous tubes of various lengths. Subsequently, the Profftella cells were lysed with detergent and dried on TEM grids without any additional processing, and the internal tubes were observed using TEM. The tubes without chemical fixation and resin embedding retained their shape even after dehydration and exposure to high vacuum conditions in the electron microscope, demonstrating their exceptional structural stability and robustness (Fig. 1B). The surface of the tubes showed a striated pattern (Fig. 1C). Their tilt series images were indicative of a right-handed helical configuration (Supplementary Movie 1). TEM analysis of Profftella cells within the syncytium, following chemical fixation, dehydration, resin embedding, and ultrathin sectioning, revealed that Profftella cells are densely packed within host cells, with minimal host cell organelles present in the spaces between the Profftella cells (Fig. 1D). The tubes exhibited a thickness of 232.00 ± 10.49 nm (Supplementary Fig. 1) and were hollow, as indicated by their low electron density (arrowheads in Fig. 1E, F). It should be noted that sample preparation steps for electron microscopy, including dehydration and embedding, can cause shrinkage of biological specimens58. Thus, the in vivo thickness of the tubes may be slightly greater than observed. To overcome the limitations of conventional 2D TEM in capturing both the overall and fine-scale morphology of Profftella, 3D reconstructions were subsequently performed.
A Isolated Profftella cells observed under an DIC microscope. B, C Tubes in ruptured but unfixed Profftella observed by TEM. D–F TEM observation of ultrathin sections of chemically fixed and resin-embedded Profftella cells and tubes. Numerous Profftella cells are packed in a syncytium of the D. citri bacteriome (D). Longitudinal view of a tube (E). Cross-sectional view of the tubes (F). p, Profftella; t, tube; cm, cell membrane of Profftella; hco, host cell organelles. Scale bars: (A) 10 μm, (B) 2 μm; (C, E and F) 200 nm, (D) 1 μm.
3D structural analysis of Profftella and its tubes
The resin-embedded bacteriome of D. citri was imaged using SBF-SEM, generating approximately 200 consecutive images, each 100 nm thick. Two datasets were acquired from the same resin block. These images provided 3D information covering a volume of 20 × 20 × 20 μm³ (Supplementary Movies 2, 3), enabling the 3D reconstruction of all Profftella cells and tubes within this volume (Fig. 2A, E, and Supplementary Movies 4, 5). A representative cell from each of the two consecutive image series is shown in Fig. 2B, F. These cells, measuring 49 μm and 81 μm in length, respectively, displayed an entangled appearance with no discernible pattern (Fig. 2C, G). The tubes within these cells varied in length (Supplementary Fig. 2, 3) and were uniformly distributed throughout the cytoplasm, without any specific directional preference. They occupied the intracellular space in a disordered, entangled manner and were occasionally observed in folded or coiled configurations (Figs. 2C-D, G-H, and Supplementary Figs. 2, 3). Despite the limited number of 200 consecutive images, 13 Profftella cells were fully reconstructed along their entire lengths (Figs. 2, 3), ranging from 2.8 μm to 81 μm (Fig. 3L). The shortest cell (2.8 μm) contained only two short tubes (Fig. 3A), while another cell with a single tube had a volume of approximately 7.2 μm³, making it the smallest cell observed (Fig. 3B). Cell volume was proportional to length, with a uniform cross-sectional area of 2.1 μm², as determined through least squares regression analysis (Fig. 3M). Based on this, the approximate diameter of the cells was calculated to be 1.63 μm.
A, E Reconstructions from 200 consecutive 100 nm-thick images obtained through SBF-SEM, showing all Profftella cells within each cubic region. Each side of the cubic blocks is 20 μm in length. Different Profftella cells are colored differently. B, F These images show representative Profftella cells where the entire cell is contained within the respective resin blocks in A and E. The planes (XY, XZ, YZ axes) of the cubic region are also displayed. C, G Enlarged views of each Profftella cell shown in B and F. The cell surfaces are displayed transparently, and numerous internal tube structures are color-coded for distinction. The centerline tracings of the cells, shown in the upper right corner of each Fig., indicate that the cells are single-stranded. The length of each Profftella cell was approximately 49 μm (C) and 81 μm (G). D, H Multiple tube structures present inside each cell shown in C and G. All tubes in D and H are individually displayed in Supplementary Figs. 2, 3.
A–K 11 fully reconstructed cells, arranged in order of increasing Profftella cell length. Each Fig. shows the cell outline along with the tube-like structures contained (different colors represent distinct tube structures). The top-right corner of each Fig. displays the centerline tracing of the respective cell. Based on the centerline tracings, the cells appear as single filaments. The red arrowheads indicate regions within the cell where there are no tubes. L Length distribution of the 11 cells shown in A–K and the 2 cells in Fig. 2C, G. M Correlation between cell length and cell volume.
Owing to the size limitations of the analysed volume, many cells could not be fully reconstructed. However, those exceeding 20 μm in length (based on the size of the consecutive images, see Fig. 2A, E) are shown in Fig. 4. The fully reconstructed cells were relatively small and confined to the limited imaging volume, allowing for complete visualization. In contrast, cells that could not be fully reconstructed were either larger or more widely distributed, preventing complete capture within the restricted volume (Fig. 4). The cell shown in Fig. 4E had a zigzag shape with four bends and a length exceeding 100 μm. The longest cell observed had a reconstructed length of 136 μm, indicating that its actual length is likely even longer (Fig. 4F). This cell uniquely displayed a single branching point, suggesting that larger cells may branch or divide into two segments (Fig. 4F, G). It also contained the highest number of tubes (43 in total) among all observed cells (Fig. 4F). At the branching point, a single long tube extended across both branches (Fig. 4H), confirming that the segmentation and rendering process did not erroneously identify two separate cells as one.
A–F Due to the data size limitations, some of the entire cells could not be fully reconstructed, but the six incomplete cells longer than 20 μm are shown. The cells are ordered by increasing length. Cross-sectional images of the block showing the ends of the Profftella cells are also included. For each cell, the centerline tracing, the cell length (L, not the full length), and the number of tubes (N) are indicated with the corresponding reconstructed images. The arrowhead indicates the branching point of the cell in F. G Magnified view of the branching point of F. H One of the tubes at the branching point extends on both branches.
All reconstructed cells contained a total of 230 tubes, with considerable variability in their lengths. Due to the 100 nm section thickness, it was impossible to measure tubes smaller than this precisely. The longest tube observed in the cell shown in Fig. 4F was approximately 45 μm in length (Fig. 5A). Notably, as cell volume increases to approximately 80–90 μm3, the internal tube occupancy increases proportionally, after which it stabilizes at around 7% of the cell volume (Fig. 5B). This is consistent with our observation of the reconstructed cell structures shown in Fig. 3A−K. In smaller cells, tube-free regions were observed at the ends (red arrowheads in Figs. 3B–C, E–F), indicating a lower tube volume fraction (Fig. 3A–G). In contrast, larger cells contained densely packed tubes distributing throughout the cytoplasm, indicating a consistent volume fraction (Figs. 3J–K and 4C–F). These observations suggest that the total volume of the tubes is constrained by cell size (Fig. 5B). Moreover, the fact that no cells entirely lacking tubes were observed in our samples may indicate that the tubes arise from the division of pre-existing ones, although the underlying mechanisms remain to be elucidated.
A Distribution of the tube length (N = 230). B Relationship between cell volume and total tube volume (N = 13). C Tomographic slices of the 8 nm thick Z-plane reconstructed by HVEM, showing the ultrastructure of the tubes within Profftella cells (see also Supplementary Movie 6). The left, middle, and right panels represent the far slice of the tubes (16th of 60 slices), the center slice of the tubes along with internal hollows (indicated by red arrows, 26th of 60 slices), and the near slice of the tubes (40th of 60 slices), respectively. D A volume-rendering image of the tube corresponding to the rectangular region in C (see also Supplementary Movie 7), showing five fibers twisted in a right-handed helical configuration. E–I TEM images of the tubes observed in 100 nm thick sections after detergent treatment, showing a longitudinal section of the tube displaying obliquely arranged sub-filaments (E), and cross-sections of the tubes (F-I) indicating that the outer wall of the tube is composed of five or six sub-filaments. Scale bars: (C) 500 nm and (E-I) 100 nm.
To analyse the finer structure of the tubes, relatively thick sections ( ~ 500 nm) were prepared for electron tomography using HVEM. Tomography data revealed that five fibers were twisted together in a right-handed helical configuration to form the tube (Fig. 5C, D and Supplementary Movies 6–8). This helical structure was also observed in unembedded bare tubes as described above (Supplementary Movie 1). The central region appeared hollow with no internal filling. To more accurately determine the number of fibers, specimens were treated with Triton X-100, fixed, dehydrated, resin-embedded, and sectioned for TEM observation (Fig. 5E–I). Cross-sectional analysis revealed that the tubes were composed of five or six fibers (Fig. 5F−I). Out of ten clearly observed cross-sections of the tubes, eight contained six fibers and two contained five fibers, suggesting that the six-fiber type is more common, while the five-fiber type is less frequent.
If a tube composed of six fibers arranged in a regular hexagonal configuration were instead formed by five fibers arranged in a regular pentagonal structure, the diameter would be expected to decrease by about 10%, amounting to more than 20 nm in a tube that measures 230 nm. This difference exceeds the standard deviation of the measured diameter distribution (10.49 nm, Supplementary Fig. 1), suggesting the possibility of bimodality, even when accounting for distortions introduced during electron microscopy sample preparation. However, the observed distribution did not display a clear bimodal pattern, as the difference was within the range of experimental variability (Supplementary Fig. 1). In the Triton X-100–treated samples (Fig. 5E–I), inter-fiber gaps were observed. Although the specific components removed by Triton X-100 remain unidentified, additional materials—possibly including ribosomes—may have originally filled these spaces. This could explain the absence of a significant diameter difference between five- and six-fiber tubes and suggests that tube diameter is relatively uniform regardless of fiber number.
Tubes contain ribosomes
FISH analysis using probes specific for the 16S rRNA of Profftella or Carsonella, along with DNA staining using Hoechst 33342, clearly distinguished between Profftella and Carsonella released from the D. citri bacteriomes (Supplementary Fig. 4). DIC microscopy demonstrated that tubes are present only in Profftella, not in Carsonella, which is consistent with our previous TEM observations7. The probe specific for Profftella 16S rRNA colocalized with the tubes in Profftella (Fig. 6A and Supplementary Movie 9), while DNA signals were distributed widely within the Profftella cells and did not align with the tubes. These results suggest that ribosomes, but not DNA, colocalize with the tubes. To assess the possibility that the probe also binds to the genomic DNA (16S rRNA gene) of Profftella, nuclease treatment was further performed before hybridization (Fig. 6B and Supplementary Movies 10, 11). DNase treatment had no effect on the colocalization of the probe with the tubes (Fig. 6B, Supplementary Movie 9), whereas RNase treatment abolished the probe signal (Fig. 6C and Supplementary Movie 10). This supports the hypothesis that ribosomes, not DNA, colocalize with the tubes, as RNase treatment is expected to degrade ribosomes by digesting rRNA, which accounts for approximately 65% of the ribosome’s molecular weight59. These results indicate that the tube is not a nucleoid but rather a structure that contains ribosomes as its components. To further investigate the structural relationship between the tubes and ribosomes, we performed TEM observations after RNase treatment. In the control group, granular structures attached to the tubes were observed (Fig. 6D), whereas in the RNase-treated group, these granular structures were absent, although the morphology of the tubes remained almost unchanged (Fig. 6E). These findings suggest that ribosomes are present within the tube but are not essential for its structural integrity.
A-D FISH images of Profftella cells: Profftella cell without nuclease treatment (A); Profftella cell with DNase treatment (B); Profftella cell with RNase treatment (C). In addition to these Alexa 488 (16S rRNA of Profftella) signals, DIC, Hoechst 33342 (DNA) images are also shown along with merged images indicating 16S rRNA colocalization with the tubes. D TEM images of the tubes without RNase treatment: cross-sectional (left) and longitudinal (right) views. E TEM images of the tubes with RNase treatment: cross-sectional (left) and longitudinal (right) views. Scale bars: (A−C) 5 μm and (D and E) 100 nm.
Discussion
This study revealed that Profftella cells contain tubes of various lengths (up to 45 μm) and numbers (ranging from 1 to 43 per cell). Each tube consisted of five or six thin fibers twisted into a right-handed helix, with a consistent diameter of approximately 230 nm along its entire length. This twisted, multi-fiber structure likely enhances the tubes’ durability and flexibility compared to a single-fiber design. It closely resembles the construction of steel wire ropes or the thick and sturdy ropes used in “tug-of-war,” where multiple thinner strands are twisted together to enhance strength, durability, flexibility, and resilience60. The tubes with this multi-fiber structure, even without fixation or embedding, retained their shape after being treated with detergent, dried, and placed under the high vacuum conditions of electron microscopes, demonstrating their remarkable stability and robustness (Fig. 1C and Supplementary Movie 1). These findings suggest that the tubes, like the cytoskeletons of eukaryotes61, may help provide mechanical stability to the highly elongated and potentially vulnerable Profftella cell while maintaining its flexibility. Additionally, the intracellular occupancy of the tubes increased proportionally with the cell volume up to 80–90 μm3 and then stabilized at approximately 7% (Fig. 5B). This indicates that tube volume is regulated in relation to cell length, implying that Profftella optimizes the tube volume to maintain structural stability across various sizes and shapes. Cytoskeletons not only provide physical support for the cell structure but also facilitate the efficient and targeted transport of various substances, including nutrients and wastes, in large cells62,63. Thus, the tubes in Profftella may also similarly serve as scaffolds for substance transport in these highly elongated Profftella cells.
This study also demonstrated that Profftella cells are highly elongated, exhibiting considerable variability in cell length, ranging from 2.8 μm to over 136 μm. This finding is consistent with our previous observation that Profftella cells are spherical when transferred from the bacteriome to the ovary but begin to elongate upon entering the oocytes, implying that Profftella is string-like within the bacteriome but transforms to a spherical shape before exiting into the hemocoel18. However, as the Profftella cells are tightly packed in the bacteriome, their precise shape and 3D arrangement remained unclear. This study resolves that uncertainty, demonstrating that the Profftella cells are highly elongated within the host bacteriome. While these morphological features are atypical from a bacteriological perspective, they are not uncommon among insect intracellular mutualists. For instance, Carsonella, the primary symbiont of psyllids15,46,64, and “Candidatus Sulcia muelleri” (Flavobacteriia: Flavobacteriales, hereafter Sulcia), an ancient obligate mutualist of various auchenorrhynchan insects65,66,67, also exhibit notable elongation in the bacteriome. Moreover, Carsonella forms spherical cells before transferring from the bacteriome to the ovary, where they enter the oocyte, forming a “symbiont ball,” which is a mosaic mass of Carsonella and Profftella18. The highly elongated shapes of Profftella, Carsonella, and Sulcia may reflect a survival strategy that prioritizes resource allocation toward symbiont functions, such as syntheses of nutrition and bioactive substances, rather than excessive investment in cell division. None of these symbionts retains the ftsZ gene14,15,66, a highly conserved gene playing a central role in bacterial cell division68,69, which may partly explain the elongated morphology of these symbionts. However, the intracellular tubular structures are found only in Profftella. The reason for this, as well as the specific Profftella genes responsible for the tube formation, are yet to be elucidated.
FISH analysis using a probe targeting the 16S rRNA of Profftella, combined with DNase/RNase treatment and DNA staining with Hoechst 33342, demonstrated that ribosomes, but not DNA, colocalize with the tubes. This rules out the possibility that the tubes are nucleoids, as seen in B. bacteriovorus56 and S. elongatus57. In B. bacteriovorus, approximately 13% of cells exhibited twisted, multi-rod-shaped nucleoid bodies arranged in a spiral formation, with length of up to approximately 1.5 μm. These nucleoids were loosely wound spirals with diameters ranging from 50 to 200 nm along their length56. In S. elongatus, the seemingly single, wavy, rod-shaped nucleoids, approximately 3 μm in length and observed only before cell division, varied in diameter from 100 to 500 nm57. In contrast, the tubes in Profftella maintain a constant diameter of 230 nm along their entire length, and their structure is clearly visible under DIC microscopy, further supporting the idea that they are not nucleoids. In Profftella, the DNA signal was broadly distributed throughout the cell (Fig. 6B). The relatively strong DNA signal in Profftella (Supplementary Fig. 4B), compared to Carsonella, suggests that Profftella harbors numerous copies of its highly reduced 460-kb genome, similar to what has been confirmed in Carsonella, which contains thousands to tens of thousands of genomic copies (160–175 kb) per cell70. TEM analysis following RNase treatment further supported the presence of ribosomes within the tubes, implying a potential involvement in gene expression. However, the analysis also indicated that ribosomes are not essential for maintaining tube structure, highlighting the need to identify the primary molecular components of the tube. Although the diameter is much smaller than that of the Profftella tube (230 nm), various helical filamentous or tubular protein-based structures—such as actin filaments ( ~ 7 nm), microtubules ( ~ 25 nm), which are found within eukaryotic cells, as well as bacterial flagella composed of flagellin (~20 nm), which extend outside the bacterial cell body—are known to exist in biological systems61. In contrast, eukaryotic flagella and cilia, which are composed of microtubules and have a comparable overall diameter (200–250 nm), do not exhibit a helical tubular architecture and are therefore not structurally analogous. Considering the possibility that the Profftella tube is also proteinaceous, future studies will be required to elucidate its molecular identity.
Methods
Insects
An established colony of D. citri, initially collected on Amami-Oshima island, Kagoshima, Japan, was maintained on the saplings of the orange jasmine, Murraya paniculata (Rutaceae). The plants covered with insect-rearing sleeves were kept in incubators at 28 °C with a 16:8 (light: dark) h photoperiod. Adult insects were collected from the plants using an insect aspirator and then caged in a plastic dish on ice for 5 min to immobilize them. They were sexed under a stereomicroscope, and bacteriomes were dissected from the abdominal hemocoel of adult females in phosphate-buffered saline (PBS: 0.8% NaCl, 0.02% KCl, 0.115% Na2HPO4, 0.02% KH2PO4).
Optical microscopy of unfixed Profftella cells
After dissection of ten D. citri individuals, bacteriomes were gently crushed with forceps and repeated pipetting in PBS, and Profftella cells released from the syncytium of the bacteriome were placed on glass slides, covered with coverslips, and observed using DIC microscopy (BX-53; Olympus, Tokyo, Japan).
TEM observation of the tubes without fixation
Profftella cells, extracted from the bacteriomes of ten D. citri individuals, were treated with detergent and observed by conventional TEM. First, bacteriomes removed from insect bodies were subjected to cell disruption by vigorous agitation in a detergent medium (0.5% Triton X-100, 5 mM MgSO4, and 100 mM HEPES-KOH pH 7.0) for 10 min at room temperature. Subsequently, some of the disrupted cells were placed directly onto TEM grids without fixation or any other additional processing to observe the tubes. The entire process, from dissection to TEM imaging, was completed within approximately 30 min to minimize structural artifacts and preserve the native morphology of the tubes. To capture the external morphology from multiple angles, tilt series images were recorded from -60° to +60° using a JEM-2100F (JEOL, Tokyo, Japan) at an accelerating voltage of 200 kV, with the results provided as Movie S1.
TEM observation of Profftella
Equal volumes of glutaraldehyde fixative (6% glutaraldehyde, 40 μM MgSO4, 4 mM sucrose, and 100 mM cacodylate buffer pH 7.0) were added to the bacteriomes dissected from ten D. citri and chemically fixed on ice for 4 h. The samples were then postfixed in OsO4 fixation solution (1% OsO4, 20 µM MgSO4, 2 mM sucrose, and 50 mM cacodylate buffer pH 7.0) for 1 h at room temperature. The samples were then dehydrated in an ethanol series, embedded in Spurr’s resin, and 100 nm sections were observed on a Hitachi H-7100 transmission electron microscope operating at 75 kV.
Sample preparation for 3D electron microscopy
The bacteriomes dissected from ten D. citri were initially treated at room temperature for 10 min with 3% glutaraldehyde in 50 mM Na-cacodylate buffer (pH 7.0), supplemented with 20 μM MgSO4 and 2 μM sucrose. Following the chemical fixation, the materials washed three times in the aforementioned buffer and were subsequently postfixed at room temperature for 30 min with 1% OsO4 in the same buffer. Fixed cells were then subjected to dehydration through a graded ethanol series (50%, 70%, 90%, 95%, 99%, and 100%) and embedded in Spurr’s resin at 70 °C for 8 h. Serial ultrathin sections (approximately 100 nm thick) were prepared using a diamond knife (EM UC7 ultramicrotome; Leica, Austria). The resulting ultrathin sections were stained with 3% uranyl acetate and lead citrate before examination utilizing an H-7100 transmission electron microscope (Hitachi, Japan) operating at 75 kV.
SBF-SEM
The resin block containing the specimens was trimmed and affixed to an aluminum rivet using conductive epoxy resin (SPI Conductive Silver Epoxy; SPI Supplies and Structure Probe, Inc., West Chester, PA, USA). Subsequently, the block was coated with gold using an ion coater. SBF-SEM (ΣIGMA/VP, Carl Zeiss Microscopy, Jena, Germany; 3View; Gatan Inc., Pleasanton, CA, USA) with a back-scattered electron detector and a diamond-knife for serial sectioning was employed for slicing and imaging the specimen. The specimen was introduced into the SBF-SEM chamber, and the block face was aligned parallel to the knife edge and brought close to the knife’s height. To mitigate charging effects, the electron microscope operated at a low accelerating voltage of 1.5 kV. Serial image series were acquired in an automated manner using Gatan Digital Micrograph software, with all images captured at a size of 8192 × 8192 pixels (pixel size = 3 nm). For SEM image acquisition, a 100-nm-thick layer was automatically removed from the block face by the knife to expose a fresh surface for imaging, and this cycle of image acquisition and block face removal was repeated. Two datasets, each consisting of 200 serial images, were acquired and aligned using the IMOD software package71. Subsequent segmentation of regions of interest was carried out using Amira version 5.4.5 (FEI Visualization Science Group, Burlington, MA, USA). Cell boundaries were segmented based on the contrast and continuity of cellular membranes observed in the serial images. Tubular structures were identified and segmented based on their distinctively high contrast and characteristic morphology, including a uniform diameter. The lengths of the reconstructed cells and tubes were manually measured using Amira, and their volumes were automatically calculated in Amira. A linear regression analysis using the least squares method was performed to evaluate the relationship between cell length and cell volume (Fig. 3M).
High-voltage electron tomography
A 500 nm-thick section collected on a single-slot grid was subjected to imaging without staining. Data acquisition was conducted using a 1,000 kV electron microscope (H1250M, Hitachi). Tilt series were captured on a 2k×2k CCD camera (FC400, Direct Electron LP) at 2° increments within a tilt range from -60° to +60°. Following image alignment, the 3D reconstruction was performed through weighted back-projection using IMOD software71. Segmentation was carried out using Amira version 5.4.5 (FEI Visualization Science Group, Burlington, MA, USA). Tubular structures were segmented based on their distinctive high-contrast morphology, characterized by right-handed helical patterns and a uniform diameter, which were consistently observed throughout the tomograms.
FISH
Bacteriomes were dissected from ten D. citri, gently crushed with forceps and repetitive pipetting to release Profftella cells, and fixed with 4% paraformaldehyde/PBS for 90 min. After washing with PBS twice, the samples were suspended with Milli-Q water, applied to glass slides, and dried on a hot plate at 40 °C. Subsequently, the hybridization buffer (20 mM Tris-HCl [pH 8.0], 0.9 M NaCl, 0.01% SDS, and 30% formamide) without probes was added and pre-incubated at room temperature for 30 min. Samples were then incubated at room temperature overnight with hybridization buffer containing 100 nM each of the probes SSDC_127247 (5’-GACCCTCTGTATGCACCATT-3’), 5’-labeled with Alexa Fluor 488 to specifically detect 16S rRNA of Profftella, and Car1 (5’-CGCGACATAGCTGGATCAAG-3’), 5’-labeled with Alexa Fluor 568 to specifically detect 16S rRNA of Carsonella. After washing three times with PBSTx (0.8% NaCl, 0.02% KCl, 0.115% Na2HPO4, 0.02% KH2PO4, and 0.3% Triton X-100), the samples were mixed with NucBlue Live ReadyProbes Reagent (Thermo Fisher Scientific, Waltham, MA, USA), a stabilized solution of Hoechst 33342 (2’-[4-ethoxyphenyl]-5-[4-methyl-1-piperazinyl]-2,5’-bi-1H-benzimidazole), a fluorescent stain for DNA. Samples were then mounted in ProLong Gold antifade reagent (Thermo Fisher Scientific) using a coverslip and were examined using a Nikon A1 laser scanning confocal microscope. Acquired images were analysed using NIS-elements AR Analysis 4.10 software (Nikon).
FISH with nuclease treatment
After fixation and washing as above, the samples were treated with DNase or RNase. DNase treatment was performed at 37 °C for 30 min using 10× DNase I Buffer, 10U of Recombinant DNase I (RNase-free, Takara, Kusatsu, Japan), and 20U of RNase Inhibitor (Thermo Fisher Scientific), followed by incubation with 0.5 M EDTA at 80 °C for 2 min. RNase treatment was performed at room temperature for ten minutes with 2.5 μg/μL of RNase A (Takara). After nuclease treatment, the samples were washed with PBS, suspended with Milli-Q water, applied to glass slides, dried, and FISH was performed as described above.
TEM observation with RNase treatment
Bacteriomes dissected from ten D. citri in HEPES buffer (100 mM HEPES-KOH [pH 7.0], 5 mM MgSO₄, and 0.5% Triton X-100) were crushed to release Profftella, and an equal volume of glutaraldehyde fixative (6% glutaraldehyde, 100 mM HEPES-KOH [pH 7.0], 4 mM sucrose, and 40 μM MgSO₄) was added for chemical fixation. Subsequent sample preparation and observation by TEM were performed using the protocol described in the “TEM Observation of Profftella” section.
Data availability
The datasets generated and/or analysed during the current study are available in the Figshare repository at https://doi.org/10.6084/m9.figshare.29371466.v2.
References
Greening, C. & Lithgow, T. Formation and function of bacterial organelles. Nat Rev Microbiol 18, 677–689 (2020).
Noble, J. M. et al. Connectivity of centermost chromatophores in Rhodobacter sphaeroides bacteria. Mol Microbiol 109, 812–825 (2018).
Cameron, J. C., Wilson, S. C., Bernstein, S. L. & Kerfeld, C. A. Biogenesis of a bacterial organelle: The carboxysome assembly pathway. Cell 155, 1131–1140 (2013).
Yang, M. et al. Decoding the stoichiometric composition and organisation of bacterial metabolosomes. Nat Commun 11, 1976 (2020).
Uebe, R. & Schüler, D. Magnetosome biogenesis in magnetotactic bacteria. Nat Rev Microbiol 14, 621–637 (2016).
Pilhofer, M., Ladinsky, M. S., McDowall, A. W. & Jensen, G. J. Bacterial TEM: New insights from cryo-microscopy. In Methods in Cell Biology vol. 96 21–45 (Elsevier Inc., 2010).
Nakabachi, A. & Suzaki, T. Ultrastructure of the bacteriome and bacterial symbionts in the Asian citrus psyllid, Diaphorina citri. Microbiol Spectr 12, e0224923 (2024).
Grafton-Cardwell, E. E., Stelinski, L. L. & Stansly, P. A. Biology and management of Asian citrus psyllid, vector of the huanglongbing pathogens. Annu Rev Entomol 58, 413–432 (2013).
Killiny, N. Made for each other: Vector-pathogen interfaces in the Huanglongbing pathosystem. Phytopathology 112, 26–43 (2022).
Nakabachi, A., Koshikawa, S., Miura, T. & Miyagishima, S. Genome size of Pachypsylla venusta (Hemiptera: Psyllidae) and the ploidy of its bacteriocyte, the symbiotic host cell that harbors intracellular mutualistic bacteria with the smallest cellular genome. Bull Entomol Res 100, 27–33 (2010).
Sloan, D. B. et al. Parallel histories of horizontal gene transfer facilitated extreme reduction of endosymbiont genomes in sap-feeding insects. Mol Biol Evol 31, 857–871 (2014).
Kwak, Y. & Hansen, A. K. Unveiling metabolic integration in psyllids and their nutritional endosymbionts through comparative transcriptomics analysis. iScience 26, 107930 (2023).
Thao, M. L. et al. Cospeciation of psyllids and their primary prokaryotic endosymbionts. Appl Environ Microbiol 66, 2898–2905 (2000).
Nakabachi, A. et al. Defensive bacteriome symbiont with a drastically reduced genome. Current Biology 23, 1478–1484 (2013).
Nakabachi, A. et al. The 160-kilobase genome of the bacterial endosymbiont Carsonella. Science 314, 267 (2006).
Nakabachi, A., Malenovský, I., Gjonov, I. & Hirose, Y. 16S rRNA sequencing detected Profftella, Liberibacter, Wolbachia, and Diplorickettsia from relatives of the Asian citrus psyllid. Microb Ecol 80, 410–422 (2020).
Nakabachi, A., Piel, J., Malenovský, I. & Hirose, Y. Comparative genomics underlines multiple roles of Profftella, an obligate symbiont of psyllids: Providing toxins, vitamins, and carotenoids. Genome Biol Evol 12, 1975–1987 (2020).
Dan, H., Ikeda, N., Fujikami, M. & Nakabachi, A. Behavior of bacteriome symbionts during transovarial transmission and development of the Asian citrus psyllid. PLoS One 12, e0189779 (2017).
Spaulding, A. W. & von Dohlen, C. D. Psyllid endosymbionts exhibit patterns of co-speciation with hosts and destabilizing substitutions in ribosomal RNA. Insect Mol Biol 10, 57–67 (2001).
Hall, A. A. G. et al. Codivergence of the primary bacterial endosymbiont of psyllids versus host switches and replacement of their secondary bacterial endosymbionts. Environ Microbiol 18, 2591–2603 (2016).
Nakabachi, A., Inoue, H. & Hirose, Y. Microbiome analyses of 12 psyllid species of the family Psyllidae identified various bacteria including Fukatsuia and Serratia symbiotica, known as secondary symbionts of aphids. BMC Microbiol 22, 15 (2022).
Nakabachi, A., Inoue, H. & Hirose, Y. High-resolution microbiome analyses of nine psyllid species of the family Triozidae identified previously unrecognized but major bacterial populations, including Liberibacter and Wolbachia of supergroup O. Microbes Environ 37, ME22078 (2022).
Maruyama, J., Inoue, H., Hirose, Y. & Nakabachi, A. 16S rRNA gene sequencing of six psyllid species of the family Carsidaridae identified various bacteria including Symbiopectobacterium. Microbes Environ 38, ME23045 (2023).
Dittmer, J. et al. Division of labor within psyllids: metagenomics reveals an ancient dual endosymbiosis with metabolic complementarity in the genus Cacopsylla. mSystems 8, 00578–23 (2023).
Nishino, K., Inoue, H., Hirose, Y. & Nakabachi, A. Microbiome of psyllids of the family Aphalaridae, including Aphalara itadori, a biocontrol agent against Reynoutria spp. Entomol Exp Appl 172, 1033–1045 (2024).
Yasuda, Y., Inoue, H., Hirose, Y. & Nakabachi, A. Highly Reduced Complementary Genomes of Dual Bacterial Symbionts in the Mulberry Psyllid Anomoneura mori. Microbes Environ 39, ME24041 (2024).
Kwak, Y. et al. Uncovering symbionts across the psyllid tree of life and the discovery of a new Liberibacter species, “Candidatus” Liberibacter capsica. Front Microbiol 12, 739763 (2021).
Sloan, D. B. & Moran, N. A. Genome reduction and co-evolution between the primary and secondary bacterial symbionts of psyllids. Mol Biol Evol 29, 3781–3792 (2012).
Ziegler, H., Pirson, A. & Zimmermann, M. H. Nature of transported substances. In Transport in plants I (eds. Zimmermann, M. H. & Milburn, J. A.) vol. 1 59–100 (Springer-Verlag, New York, 1975).
Sandström, J. & Moran, N. How nutritionally imbalanced is phloem sap for aphids? Entomol Exp Appl 91, 203–210 (1999).
Yamada, T., Hamada, M., Floreancig, P. & Nakabachi, A. Diaphorin, a polyketide synthesized by an intracellular symbiont of the Asian citrus psyllid, is potentially harmful for biological control agents. PLoS One 14, e0216319 (2019).
Nakabachi, A. & Okamura, K. Diaphorin, a polyketide produced by a bacterial symbiont of the Asian citrus psyllid, kills various human cancer cells. PLoS One 14, e0218190 (2019).
Nakabachi, A. & Fujikami, M. Concentration and distribution of diaphorin, and expression of diaphorin synthesis genes during Asian citrus psyllid development. J Insect Physiol 118, 103931 (2019).
Tanabe, N. et al. Diaphorin, a polyketide produced by a bacterial symbiont of the Asian citrus psyllid, inhibits the growth and cell division of Bacillus subtilis but promotes the growth and metabolic activity of Escherichia coli. Microbiol Spectr 10, e0175722 (2022).
Izu, T., Uchida, N., Takasu, R. & Nakabachi, A. Antibacterial spectrum of diaphorin, a polyketide produced by a bacterial symbiont of the Asian citrus psyllid. J Invertebr Pathol 211, 108309 (2025).
Takasu, R., Yasuda, Y., Izu, T. & Nakabachi, A. Diaphorin, a polyketide produced by a bacterial endosymbiont of the Asian citrus psyllid, adversely affects the in vitro gene expression with ribosomes from Escherichia coli and Bacillus subtilis. PLoS One 18, e0294360 (2023).
Takasu, R., Izu, T. & Nakabachi, A. A limited concentration range of diaphorin, a polyketide produced by a bacterial symbiont of the Asian citrus psyllid, promotes the in vitro gene expression with bacterial ribosomes. Microbiol Spectr 12, e0017024 (2024).
Nakabachi, A., Ishikawa, H. & Kudo, T. Extraordinary proliferation of microorganisms in aposymbiotic pea aphids, Acyrthosiphon pisum. J Invertebr Pathol 82, 152–161 (2003).
Nikoh, N. & Nakabachi, A. Aphids acquired symbiotic genes via lateral gene transfer. BMC Biol 7, 12 (2009).
Shigenobu, S. et al. A full-length cDNA resource for the pea aphid, Acyrthosiphon pisum. Insect Mol Biol 19, 23–31 (2010).
Tamborindeguy, C. et al. A genomic analysis of transcytosis in the pea aphid, Acyrthosiphon pisum, a mechanism involved in virus transmission. Insect Mol Biol 19, 259–272 (2010).
Nakabachi, A., Ishida, K., Hongoh, Y., Ohkuma, M. & Miyagishima, S. Aphid gene of bacterial origin encodes protein transported to obligate endosymbiont. Current Biology 24, R640–R641 (2014).
Nakabachi, A. Horizontal gene transfers in insects. Curr Opin Insect Sci 7, 24–29 (2015).
Nikoh, N. et al. Bacterial genes in the aphid genome: absence of functional gene transfer from Buchnera to its host. PLoS Genet 6, e1000827 (2010).
Nakabachi, A. & Miyagishima, S. Expansion of genes encoding a novel type of dynamin in the genome of the pea aphid, Acyrthosiphon pisum. Insect Mol Biol 19, 165–173 (2010).
Nakabachi, A. Mutualism revealed by symbiont genomics and bacteriocyte transcriptomics. in Insect Symbiosis (eds. Bourtzis, K. & Miller, T. A.) vol. 3 163–204 (CRC Press, New York, 2008).
Nakabachi, A. & Ishikawa, H. Polyamine Composition and Expression of Genes Related to Polyamine Biosynthesis in an Aphid Endosymbiont, Buchnera. Appl Environ Microbiol 66, 3305–3309 (2000).
Nakabachi, A. & Ishikawa, H. Expression of host S-adenosylmethionine decarboxylase gene and polyamine composition in aphid bacteriocytes. Insect Biochem Mol Biol 31, 491–496 (2001).
Himler, A. G. et al. Rapid spread of a bacterial symbiont in an invasive whitefly is driven by fitness benefits and female bias. Science 332, 254–256 (2011).
Nakabachi, A. et al. Horizontal gene acquisition of Liberibacter plant pathogens from a bacteriome-confined endosymbiont of their psyllid vector. PLoS One 8, e82612 (2013).
Jain, M., Fleites, L. A. & Gabriel, D. W. A small Wolbachia protein directly represses phage lytic cycle genes in ‘Candidatus Liberibacter asiaticus’ within psyllids. mSphere 2, e00171–17 (2017).
Kruse, A. et al. Combining ’omics and microscopy to visualize interactions between the Asian citrus psyllid vector and the Huanglongbing pathogen Candidatus Liberibacter asiaticus in the insect gut. PLoS One 12, e0179531 (2017).
Chu, C., Hoffmann, M., Braswell, W. E. & Pelz-Stelinski, K. S. Genetic variation and potential coinfection of Wolbachia among widespread Asian citrus psyllid (Diaphorina citri Kuwayama) populations. Insect Sci 26, 671–682 (2019).
Kaji, T., Kakui, K., Miyazaki, N., Murata, K. & Palmer, A. R. Mesoscale morphology at nanoscale resolution: Serial block-face scanning electron microscopy reveals fine 3D detail of a novel silk spinneret system in a tube-building tanaid crustacean. Front Zool 13, 14 (2016).
Chihara, A. et al. A novel capsid protein network allows the characteristic internal membrane structure of Marseilleviridae giant viruses. Sci Rep 12, 21428 (2022).
Butan, C. et al. Spiral architecture of the nucleoid in Bdellovibrio bacteriovorus. J Bacteriol 193, 1341–1350 (2011).
Murata, K., Hagiwara, S., Kimori, Y. & Kaneko, Y. Ultrastructure of compacted DNA in cyanobacteria by high-voltage cryo-electron tomography. Sci Rep 6, 34934 (2016).
Song, C. & Toshinobu, S. Improved preservation of organelles in Paramecium bursaria by freeze-substitution with glutaraldehyde and osmium tetroxide. J. Electr. Microsc. Technol. Med. Biol. 27, 1–8 (2013).
Kurland, C. G. Molecular characterization of ribonucleic acid from Escherichia coli ribosomes. J Mol Biol 2, 83–91 (1960).
Peng, Y. et al. Friction and wear of multiple steel wires in a wire rope. Friction 11, 763–784 (2023).
Fletcher, D. A. & Mullins, R. D. Cell mechanics and the cytoskeleton. Nature 463, 485–492 (2010).
Woodhouse, F. G. & Goldstein, R. E. Cytoplasmic streaming in plant cells emerges naturally by microfilament self-organization. Proceedings of the National Academy of Sciences 110, 14132–14137 (2013).
Guedes-Dias, P. & Holzbaur, E. L. F. Axonal transport: Driving synaptic function. Science 366, 1979 (2019).
Abe, H., Aikawa, C., Nakabachi, A., Miyakoshi, M. & Maruyama, F. New insights from infection-specific gene expression network. Nihon Saikingaku Zasshi 69 (2014).
Moran, N. A. Symbiosis as an adaptive process and source of phenotypic complexity. Proceedings of the National Academy of Sciences 104, 8627–8633 (2007).
McCutcheon, J. P., McDonald, B. R. & Moran, N. A. Origin of an Alternative Genetic Code in the Extremely Small and GC–Rich Genome of a Bacterial Symbiont. PLoS Genet 5, e1000565 (2009).
Moran, N. A., McCutcheon, J. P. & Nakabachi, A. Genomics and evolution of heritable bacterial symbionts. Annu Rev Genet 42, 165–190 (2008).
Egan, A. J. F., Errington, J. & Vollmer, W. Regulation of peptidoglycan synthesis and remodelling. Nat Rev Microbiol 18, 446–460 (2020).
Cameron, T. A. & Margolin, W. Insights into the assembly and regulation of the bacterial divisome. Nat Rev Microbiol 22, 33–45 (2024).
Nakabachi, A. & Moran, N. A. Extreme Polyploidy of Carsonella, an Organelle-Like Bacterium with a Drastically Reduced Genome. Microbiol Spectr 10, e0035022 (2022).
Kremer, J. R., Mastronarde, D. N. & McIntosh, J. R. Computer Visualization of Three-Dimensional Image Data Using IMOD. J Struct Biol 116, 71–76 (1996).
Acknowledgements
We thank Ms. Sachiko Yamada for her valuable assistance in segmenting the SBF-SEM data. This work was supported by the Japan Society for the Promotion of Science (https://www.jsps.go.jp) KAKENHI (grant numbers 21687020, 26292174, 20H02998 and 25K02023 to AN), the Collaborative Study by High Voltage Electron Microscopy Program (2015-502, 2016-502) of National Institute for Physiological Sciences to AN, and a National Research Foundation of Korea (NRF) grant RS-2024-00440289 to CS. The funders had no role in the study design, data collection and analysis, decision to publish, or manuscript preparation.
Author information
Authors and Affiliations
Contributions
C.S., J.M., K.M., T.S., and A.N. performed the research; C.S., T.S., and A.N. wrote the manuscript; all authors reviewed and approved the final version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Song, C., Maruyama, J., Murata, K. et al. Enigmatic tubular ultrastructure in the bacterial defensive symbiont of the Asian citrus psyllid Diaphorina citri. npj Imaging 3, 44 (2025). https://doi.org/10.1038/s44303-025-00107-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s44303-025-00107-w