Abstract
To most, if not all, species, the food availability shows both daily and seasonal fluctuations in its timing, quality, and abundance. Hence, coupled with the appetite (urge to eat), it results in species-specific foraging (eating or feeding) patterns, an ecological determinant of almost all the aspects of behavior and physiology in animals. The feeding times control mutually inclusive physiological events by affecting metabolic homeostasis (a direct effect) and/ or by their synchronization effects on the underlying circadian rhythms (an indirect effect). Experiments have shown that food intake at an inappropriate time of the day (i.e., at a wrong time relative to the internal circadian clock) negatively affects the circadian homeostasis and consequently the behavior, metabolism and reproduction in animals. The food availability times as a conditioning environment influence epigenetics, evidenced by chromatin activation/ silencing at genome levels in several species. Here, we review briefly the experimental evidence largely from birds and mammals to provide insights into when and how daily feeding times affect the clock-controlled behavior, metabolism and reproductive fitness in diurnal species.
Similar content being viewed by others
Introduction
The timing of food consumption (feeding or eating times) is a trade-off between daily fasting periods (interval between feeding times) and the foraging cost (time and effort required to gain food access). Thus, to maintain metabolic homeostasis, there are individual and/ or species-specific foraging patterns to ensure feeding when food access is consistent and adequate food access1. However, to most species, food access shows both daily and seasonal fluctuations in when it is available (timing), how much it is available (abundance), and what is available (quality) in abundance. The temporal variation in the food availability/ feeding can be an important driver of both clock and non-clock effects. These involve cascading effects on the hypothalamus releases, which consequently impact multiple mechanistic pathways downstream controlling many biological functions from growth and development, energy homeostasis, physiology, to metabolic and reproductive fitness2.
The role of feeding times in metabolic homeostasis is most directly evidenced when food access is limited (restricted) to a specific or preferred eating time-window during the day. Experimentally, using a food-restriction protocol based on ‘when’ food is available during the day, and/ or ‘how much’ energy is available from food accessed shows this. In the former, the food access is ad lib (ad libitum, at one’s pleasure; Latin) but only for a timed period; this is termed time-restricted feeding (TRF)3. The latter involves a calorie-restricted feeding in which food eaten provides a significantly reduced (20–40%) calorie content, as compared to that is available from an ad-lib feeding. Experiments using both these feeding protocols have shown beneficial effects on food consumption4,5. For example, the nocturnal rats fed a high-fat diet in a TRF protocol were neither obese nor they showed any obesity-related metabolic disorder despite consuming the same calories as in ad lib feeding6. Similarly, the calorie-restricted feeding enhanced both, the reproductive activity and reproductive life span in turkey hens, Meleagris gallopavo7.
The surrounding environment can serve as a conditioning agent, and induce chromatin activation/ silencing at genome levels8,9,10. This is termed as the epigenetic modification, in which the environmental factors influence the expression of genes in both regulatory (e.g., hypothalamus) and effector (e.g., liver, gonads) tissues without changing the nucleotide sequences; this results in an altered phenotype11,12,13,14. The emerging evidence suggests a functional linkage of the quality, quantity, and timing of food access with the epigenetic modification in both mammals and birds2,15,16,17,18.
The present review focuses mainly on research that were aimed at investigating how the feeding times affect the clock-controlled behaviors, physiology, metabolism and reproductive performance both at the phenotype and molecular levels in diurnal vertebrates. The discussion centers on results accumulated mainly from the two lines of evidence. The first line of evidence includes results from the experiments in which a food restriction period is imposed during a normal 24-h light-darkness (LD) cycle. Such a daily time-restricted feeding protocol has been used extensively in investigating the circadian rhythm effects of food consumption in mammals—rat, mice, and human6,19,20,21,22,23,24, and zebra finch, Taeniopygia guttata3,25,26,27. The second line of evidence is from the experiments in which feeding time is altered because of disruption in the temporal partitioning between the day and night. This occurs in an urbanized environment in which an increasing usage of artificial light at night blurs the clear separation between light dark components of the 24-h day28,29. The resultant tampering of the subjective interpretation of the day and night components day impacts daily feeding pattern in both nocturnal rodents and birds2,30,31. At the end, we also present a perspective for the ecological and evolutionary significance of the food availability and feeding times in higher vertebrates, including humans.
Daily eating pattern (24-h feeding–fasting periods) is a circadian clock-controlled behavior
Daily cycles in the light and darkness periods result in corresponding cycles of other environmental variables, such as temperature: daytime is always warmer than the night, and winters with short daylengths are cooler than the summers with longer daylengths. Fundamental to survive in such a cyclic environment is the ability of organisms to acquire food when it is available with a lesser effort during its active period (awake; feeding time) and store (assimilate) a portion of it for utilization during the rest period (sleep, no-feeding or fasting time). Not surprisingly, in both diurnal and nocturnal animals, the daily food consumption (foraging) occurs in regular and recurring feeding–fasting cycles, coinciding with 24-h cycles of active and rest periods32,33,34. Diurnal animals eat exclusively during the daytime when they are active (or awake), and fast (starve) mostly at night when they are at rest (or sleep); hence, there is a complete phase overlap of the feeding and activity periods (Fig. 1a). Converse is true of a nocturnal species, which is at rest (sleep) and fasts during the day, and eats during the awake/ active period at night. Interestingly, however, the feeding and activity periods are almost in opposite phase profiles in diurnal migrant passerines (Order: Passeriformes) in the migration period when birds fly at night35,36.
a Representative double plotted 24-h patterns (actograms) of locomotion and feeding (eating) activities in European starlings (Sturnus vulgaris) sequentially exposed to bright and dim LD (12 h light: 12 h darkness; LDbright, 500:0.2 lux; LDdim, 2: 0.2 lux) and constant dim light (LLdim, 0.2 lx) conditions, as mentioned on the right-hand side of the diagrams. The corresponding profiles of plasma melatonin levels are interposed within the feeding actogram. b Double plotted 24-h pattern of feeding actogram in a starling exhibiting temporal association between feeding and melatonin rhythms. This figure is based on results included in a publication of Kumar et al.38, with permission; for reference details please see the reference section.
The metabolic homeostasis requires a consistent energy supply from the daily feeding–fasting cycle, necessitating the coincidence of appetite with the foraging and food availability periods. This is achieved by the internal circadian (Latin: circa = about; dies = day) clock(s), which is(are) expressed as circadian rhythms. Like locomotion (activity), which is a reliable marker of the circadian clock activity, the daily feeding—no feeding (feeding–fasting) cycles conform to a circadian rhythm35,37 (Fig. 1). It is entrained to a 24 h period under the normal LD environment, and free-runs with a circadian period under constant conditions, 24-h dim light (LLdim) or complete darkness, DD (Fig. 1)34,37,38.
The circadian rhythm in feeding maintains a stable phase relationship with the other circadian activities, as evidenced by a high correlation coefficient between the locomotion (activity), feeding and melatonin secretion patterns across the time and light conditions in European starlings, Sturnus vulgaris38 (Fig. 1a). Interestingly, the rhythm in melatonin secretion is more closely linked to feeding than to the locomotion, as shown in starlings (Fig. 1b), suggesting a crucial role of daily feeding times in the internal organization of bodily rhythms2,38. However, in an experiment on starlings, Kumar et al.39 demonstrated that the circadian clock(s) governed mutually coupled daily behavioral rhythms independently. Here, starlings were exposed to LD cycles composed of 12 h light period coupled with steadily lengthening and shortening darkness periods by 4 min per day until T (period of LD cycle) equaled 26.5 h and 21.5 h, respectively. This resulted in alterations in the phase relationships of the underlying circadian oscillators because of differences in their rates of entrainment to imposed varying Ts. When T exceeded the limit of entrainment, the rhythms in locomotion and feeding broke loose from the LD cycle, and subsequently became out of phase from each other and free-ran with their own circadian periods39.
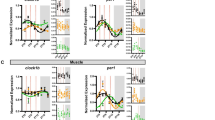
The relative illumination of the light and darkness periods of an LD cycle influences the phase relationships between circadian rhythms. Various rhythms are closely coupled with each other as long as the underlying circadian oscillators subjectively interpret and clearly differentiate between light and dark phases of the 24-h day, based on the illumination intensity and/ or photophase contrast40,41. When the temporal partitioning between light and dark components of the 24-h day is blurred, for example by exposure to dim light at night (dLAN), or when it is absent in a no-night (LL, constant light) environment, there is the misalignment (desynchrony) in circadian rhythms governing the behavior and physiology31,42,43,44,45,46,47,48,49. Interestingly, Batra et al.45,46 found changes in the behavior, physiology, and hepatic expression of core clock genes in female zebra finches subjected to a dLAN environment (Fig. 2a-i).
The figure presents a comparison of different parameters related to the behavior (sleep, feeding; left panel), physiology (middle panel), and hepatic clock gene expressions (right panel) between female zebra finches exposed to LD (12 h light at ~150 lux coupled with 12 h darkness at 0 lux (LD) or with dim light at night (~5 lux, dLAN). a plasma oxalate levels, a marker of sleep debt. b Nocturnal feeding duration. c Nocturnal feeding bouts; i.e., feeding frequency. d Food intake. e Body mass. f Subjective score of body fattening. g–i Hepatic mRNA expression of clock genes (Per2, Bmal1 and Rev-erb b). Note significant effects under the dLAN in different parameters at p < 0.05 level, as indicated by an asterisk, and changes in the phase and/ or amplitude of daily mRNA oscillations clock genes. Interestingly, food intake was higher at night under the dLAN but the total 24-h food intake did not differ significantly between LD and dLAN conditions. The figure is drawn based on results from our laboratory included in publications by Batra et al.45,46,48 (for reference details see the reference section).
An interesting aspect of the circadian feeding behavior that is largely unexplored is that it can be described along two separate dimensions; circadian rhythm and diurnal patterns. The circadian rhythm refers to the recurring feeding–fasting cycles along a 24-h time scale, while a diurnal pattern refers to the structure of the repeating feeding behavior (e.g., low and high food intake times) within the 24-h day with possible species and individual differences50. A diurnal feeding pattern could represent the response to both external and internal changes (stressors); for example, diurnal pigs faced with heat stress, shift much of their feeding hours during the night51. Similarly, the migratory passerine birds show seasonal differences in their diurnal feeding behavior in and out of the migration period2,52.
Food availability and feeding times: effects on metabolism
Alternating feeding and fasting periods coincide with the anabolic and catabolic phases of metabolism, respectively, as a consequence of the synchrony between underlying circadian rhythms. This is disrupted by an irregular availability of food, eating at inappropriate time relative to the circadian timing, and/ or by the dissociation between daily cycles of wake-sleep and feeding–fasting. The resultant circadian misalignment effects impacts the glucose and lipid homeostasis53. A delayed mealtime causes a comparable phase delay in the circadian rhythm of plasma glucose levels in humans under a constant routine condition54,55. The most common symptom of such a disruption is obesity and related health issues in humans56,57.
The TRF paradigm sets temporally regular and recurring fasting periods, and consequently affects the metabolic homeostasis. For example, in mice under 12 h light:12 h dark, feeding during light period (biological night and rest phase) impairs the metabolic homeostasis and results in obesity although neither the total caloric intake was different from those fed only at night (the biological day and active phase) nor was the difference in the physical activity between two groups6,19,20,21. Notably, even in a TRF condition, the timing of food access is crucial; for example, feeding at the wrong time during the light period negatively affects the body metabolism and reproduction in diurnal zebra finches maintained under 12 h light: 12 h darkness, 12:12D (Figs. 2, 3). More specifically, Kumar and colleagues have reported that a 4 or 5-h food availability period aligned with light off time caused negative reproductive effects, compared to when it was aligned with the light on time in blackheaded buntings, Emberiza melanocephala58, house sparrows, Passer domesticus59 and zebra finches3.
Left panel describes the experimental design. Zebra finch pairs hatched and raised indoors under 12 h light: 12 h darkness (L= ~150 lux; D = 0 lux) were subjected to a 4-h food restriction in the morning (FA-M: hour 0-4, aligned with light on time – hour 0) or evening (FA-E: hour 8–12, aligned with light off time), with controls on food ad libitum (FAL) for about a year. The middle panel presents the time-dependent effects of food restriction on measures of reproduction (top two figures), and a correlation of plasma T (testosterone) levels in males with the offspring survival (bottom figure). The right most panel shows food restriction effects on hypothalamic expression Hdac genes linked with the epigenetic regulation. The alphabets on bars indicate comparison between three feeding groups; same alphabet – no difference; different alphabets—a significant difference at p < 0.05 level. The figure is drawn based on results from our laboratory included in publications by Mishra and Kumar3, and Mishra et al.27; for reference details see the reference section.
A disruption in the temporal partitioning of an LD environment (e.g., dLAN) affects the metabolic homeostasis60,61,62. Batra et al.45,46 investigated this in diurnal zebra finches exposed to 12 L:12D with the dLAN. In a temporally disrupted dLAN environment, an exclusively daytime-eating diurnal zebra finches show disruptions in its nocturnal sleep resulting into sleep dept as indicated by reduced plasma oxalate levels (Fig. 2a), daily feeding patterns with eating also at night without any significant change in the 24-h total food intake (Fig. 2b–d; Batra et al., 2019). As a result, the zebra finches undergo metabolic impairment, and exhibit fattening and body mass gain (Fig. 2e, f), and hepatic lipid accumulation45,46,47,63. There were concurrent negative effects in the hepatic expression of genes involved in carbohydrate and fat metabolism (Fig. 4), suggesting an enhanced gluconeogenesis and impaired fatty acid synthesis45.
Left panel shows the effects on parameters of reproduction (competence, performance and fitness) in zebra finch pairs subjected to a 4-h food restriction (for experimental details please refer to the Fig. 3). The right panel compares the metabolic effects in female zebra finches fed only during the 12 h light period with those fed ad libitum under the dLAN environment. The upward and downward arrows indicate significant increase and decrease, respectively, in the parameter assayed at p < 0.05 levels under dLAN, as compared to the LD condition. The figure is drawn based on results from our laboratory included in publications by Mishra and Kumar3, and Batra et al.48 (for reference details see the reference section).
Much, if not all, of the negative feeding-induced effects result from the functional impairment of the liver. This is reflected in a significant cellular accumulation of lipids, and negative changes in the expression of genes associated with the glucose and fat metabolism in the liver (e.g., glucose-6-phosphatase, g6pc; forkhead box-O 1, foxo1; sirtuin1, sirt1; Fig. 4)45,64,65,66. Despite the evidence for transcriptional changes, it is not unlikely that food-induced effects are post-transcriptional or they involve post-translational modification(s) of gene-encoded proteins67. They may also represent a cumulative response of multiple genetic pathways involved in the regulation of body metabolism and energy homeostasis45,46,47,48,63.
Furthermore, Batra et al.48 addressed a key question whether the restriction of feeding during ‘normal’ (i.e., during the daytime in a diurnal species) would mitigate the negative effects of eating at the ‘wrong’ time of day in a dLAN environment. To answer this, zebra finches under dLAN were provided ad lib food during the 12-h light period, with controls on ad lib food all the 24-h day. In spite of similar food intake in both conditions, the metabolic impairment was significantly reduced in birds on restricted feeding period; i.e., fed only during the 12-h light period. This was evidenced by a significant reduction in the fat accumulation and body mass gain, and the reversal of changes in hepatic expression of genes involved in the carbohydrate and fat metabolism48.
Food availability and feeding times: effects on sleep
The eating time adversely affects the wake-sleep pattern in a diurnal species. The midnight eating is known to disrupt sleep (hence frequent awakenings), resulting in short sleep duration in humans68. Although there may not be a significant adverse effect on the overall night sleep architecture, the dinner timing could influence the distribution of sleep stages in humans69. Kumar and his colleagues have investigated this further in experiments on non-model animals, the Indian house crow and zebra finch, under the dLAN or LL environment42,44,46,48,70. In a study on zebra finches, the effects of dLAN on night sleep were assessed by behavioral and physiological (plasma oxalate levels, a biomarker of sleep disruption)71 assays, as well as by the measurement of hypothalamic expressions of regulatory genes involved in sleep (cytokine pathway genes: toll-like receptor 4, tlr4; tumor necrosis factor-alpha, tnf-α; interleukin-1 beta, il-1β; nitric oxide synthase, nos; calcium-dependent pathway genes: calcium/ calmodulin dependent protein kinase II, camk2; salt-inducible kinase 3, sik3; NMDA receptor subtype 3a, nr3a), and awake (cholinergic muscarinic receptor subunit 3, achm3) states46. Zebra finches showed shorter sleep bouts, increased sleep latency, reduced total night sleep, reduced plasma oxalate levels, along with changes in the phase and amplitude of 24-h rhythms of circadian clock genes46 (Fig. 2a). The subsequent study addressed whether feeding at night, not the dLAN alone, was causal to sleep disturbances in zebra finches. To decipher this, Batra et al.48 compared sleep effects between ad lib and restricted (12-h light period only) feeding conditions in zebra finches under the dLAN environment. Although, the food consumption was similar in both feeding conditions, birds with food access during the 12-h light period alone showed the reversal of dLAN-induced effects on sleep, along with that in daily activity behavior48. A positive sleep effect was evidenced by increased bout lengths and total duration, as well as increased plasma oxalate levels48. Consistent with this, the hypothalamic mRNA expressions of sleep promoting sik3 and camkii genes were higher, and that of the awake promoting achm3 gene was lower under dLAN in zebra finches with 12-h daytime food access than in those with 24-h food access48.
Food availability and feeding times: effects on reproduction
Abundant food supply is absolutely critical for the reproductive success, which is a cumulative result of the reproductive competence (mature gonads in paired mates), performance (offspring survival) and fitness (maximize the profitable reproductive success) of breeding pairs. This is widely seen among seasonally breeding birds and mammals when they complete gonadal maturation in anticipation of the reproductive season, and mate at a time when the food supply will be most abundant for the offspring survival.
Reproductive competence
The end point of the reproductive competence is gonadal maturation, which culminates in the mating of breeding pairs, and subsequently egg laying or pregnancy, as appropriate. The hypothalamus-pituitary (HP) axis via changes in the follicle stimulating and luteinizing hormone secretions controls the maturation of gonads, and concurrently enhances gonadal hormones; testosterone (T) and estradiol (E2) in male and female parents, respectively3,72,73. Increasing evidences have shown the feeding time effects on several parameters defining the reproductive competence. For example, the food restriction reduced plasma T levels in male zebra finches3,74. Mishra and Kumar3 carried out a more detailed study on 4-h TRF-induced effects on the reproductive competence in zebra finch breeding pairs maintained on the 12-h day (12 L:12D; Fig. 3). As compared to the pairs fed for 4 h in morning (hour 0-4; hour 0 = light on), the pairs fed for 4 h in the evening (hour 8-12) (hour 0 = light on) had reduced circulating levels of T in males and E2 in females, and mesotocin (a measure of affability, i.e., eagerness to engage in sexual activity) in both sexes3. Notably, plasma T and E2 levels were significantly correlated with the reproductive competence; for example, females with low E2 levels had a delayed egg laying3 (Fig. 3). Thus, TRF adversely affected the reproductive health and competence in zebra finches3. (Similar to continuously breeding zebra finches, the long-day breeding blackheaded buntings (Emberiza melanocephala) and house sparrows (Passer domesticus) fed for 5 h in the evening (hour 11 – 16, hour 0 = light on), but not in morning (hour 0 – 5), showed reduction almost by half in the testis maturation under 16 h long days58,59. Food restriction also delayed and/or led to a sub-maximal gonadal maturation under stimulatory photoperiods in European starlings75 and Abert’s Towhees (Melozone aberti)76.
Reproductive performance
Kumar and his colleagues demonstrated the effects of the daytime food access timings on reproductive performance in zebra finch pairs hatched and raised under 12 L:12D and were subjected for about a year to a TRF regime such that they received food only for 4 h either in morning (hour 0–4, FA-M) or in the evening (hour 8–12, FA-E). The results on the measurers of reproductive performance from TRF pairs were compared with control pairs on an ad lib feeding regimen, FAL (Figs. 3,4). The reproductive fecundity of FA-M pairs was similar to the FAL pairs, but the egg quality was poor resulting in the lower-quality offspring; for example, the offspring from FA-M pairs were smaller in size, had poor skeleton growth, and hence when adult they were leaner and weighed lower than those from the ad lib feeding pairs3. In contrast, the FA-E pairs had significantly delayed the egg laying but the eggs were of a better quality3. The resultant offspring from FA-E pairs although fewer in number were larger in size, had a good skeletal growth, and hence when adult they weighed heavier than those from FA-M pairs (Fig. 4).
Prabhat et al.47 suggested that a TRF regimen could lead to homeostatic adaptation in zebra finches. Birds exposed to 12 L:12D were subjected for 1- or 3 weeks to a 4-h evening TRF (hour 8-12 of 2-h day); the control group were on ad lib feeding. Compared to ad lib, testes were smaller in size and plasma T levels were lower after 1, but not after 3 weeks of the evening TRF. There were also concomitant changes in the mRNA expression of reproductive genes47. In particular, there were concurrent changes in the mRNA expression of genes coding for the gonadotropin-releasing hormone (GnRH) in hypothalamus, and of receptors for androgen (AR) and estrogen (ER-alpha) in both hypothalamus and testes. Importantly, the negative molecular effects after week 1 were alleviated to a considerable extent by week 3 of the TRF experiment47.
Reproductive fitness
Reproductive fitness determines the ability of organisms to carry their progeny. A measure of this is the reproductive success, which is a function of the relationship between the fertility and fecundity77. To optimize fitness, the breeding pairs could respond to a food availability environment limited in time and space, in four possible ways. First, the egg size is reduced so that reproductive fecundity is increased (smaller size → more eggs → more offspring → more survivors). Secondly, much of the energy available into egg production is invested to increase the number of offspring; however, this might reduce energy available for the post-egg developmental stages, and hence affect the offspring survival probability. Thirdly, the egg production is delayed until an adequate food reserve is available, although this may result in fewer nesting attempts during the breeding season. Lastly, the pairs avoid breeding during an inadequate food supply period, although again this is a risky proposition with a low breeding probability in the subsequent season. Irrespective of these probable responses, breeding pairs when faced with nutritional deficits selectively optimize their reproductive fitness (offspring fitness) by differentially allocating the available nutritional resource to eggs and feeding the hatchlings. In fact, the nutritional state of mother affects, the size and number as well as macronutrients (e.g., protein and lipid content) of the eggs laid78.
Thus, there is an adaptation to limited food availability environment, which is reflected in the trade-off between quantity (how many offspring produced and/ or survived) and quality (how good offsprings are) of a reproducing species. Mishra and Kumar3 demonstrated the food-availability-time-dependent quantity-quality trade-off in zebra finches. Absence of food in the morning compromised the reproductive fecundity but not the offspring quality; on the other hand, a similar food-absence in the evening compromised the offspring quality (skeletal growth and overall size) but not the reproductive fecundity in zebra finches exposed to 12L:12D and subjected to a 4-h TRF in morning or evening3. The offspring growth and health were severely compromised in morning but female offsprings were in the better health, suggesting a sex-biasness in food provisioning by breeding parents3.
Mechanism(s) underlying food availability effects on metabolism and reproduction
Light promotes a diurnal species to be alert and active, and forage exclusively during the daytime; conversely, the presence of light stimulates a nocturnal species to seek shelter, become inactive and even sleep, and hence it forages during darkness of the night. Endogenous circadian clocks achieve such a close coupling of the feeding–fasting period with 24-h LD cycle2,37. At the mechanistic level, the food and feeding times exert both direct (via hypothalamic machinery) and indirect (via internal circadian rhythms and epigenetic modifications) effects on the regulatory processes controlling behavior and physiology at multiple levels. Here, along these two lines of experimental approaches, we will discuss the evidence derived mainly from TRF studies in a few species.
Direct effect: involvement of hypothalamus
Hypothalamus is the key brain region regulating appetite and energy homeostasis. Distinct hypothalamic neuronal populations sense the nutrient status, and integrate signals emanating from other brain area, and peripheral tissues to control the food intake and energy homeostasis during the intermittent feeding intervals79. Thus, there is a direct feeding effect on hypothalamus, which can be much discernible in the face of a restricted food availability since there is a cost to forage and store sufficient food especially in a given time period to meet the metabolic costs80.
Hypothalamic pathways and metabolism
Many animal studies have evidenced the functional role of hypothalamus in feeding processes81,82,83. The hypothalamic arcuate nucleus, in particular, contains two populations of neurons expressing the orexigenic (appetite stimulating) neuropeptide Y (NPY) and agouti-related peptide (AgRP), and anorectic cocaine and amphetamine-related transcript (CART) and pro-opiomelanocortn (POMC, producing alpha-MSH). Both these sets of cells are leptin-responsive neurons projecting to the melanocortin 4 receptors; hence, they form the hypothalamic leptin-melanocortin pathway and regulate food intake processes, and consequently the metabolism and body weight in animals, and perhaps humans84,85. Experimentally, Singh et al.86 showed in zebra finches that in interaction with NPY, the CART stimulated appetite loss, and hence it was involved in the energy balance when birds were subjected to varying fasting-refeeding regimes. In another study on zebra finches, however, a 4-h TRF in morning or evening did not alter the hypothalamic immunoreactivity for NPY and CART in both sexes under the 12L:12D, which could be possibly suggest that birds were not completely starved if given food for 4 h in a 12-h day3,25. Among these metabolic peptides, the role of NPY is much widely investigated, and reviewed frequently87. The distribution of NPY and CART has been reported in several brain areas including the telencephalon and hind brain of both birds and mammals, implicating these two as multifaceted peptides with roles extending from the metabolic syndrome (appetite control, food intake and energy homeostasis), daily and seasonal timing, anxiety to reproduction86,87,88,89,90.
Hypothalamic pathways and reproduction
There is a strong association between energy balance (metabolic homeostasis) and energetically expensive reproduction. Hypothalamic neurons expressing NPY/ AgRP and CART/ alpha-MSH peptides are the critical regulatory sites for both metabolism and reproduction91,92. Anatomically, NPY fibers lie in close proximity with GnRH-I (gonadotropin releasing hormone I, secreted mainly from the hypothalamic preoptic area and regulates gonadal maturation), GnRH-II (synthesized mainly in midbrain region and regulates reproductive behavior) and GnIH (gonadotropin-inhibiting hormone, secreted from hypothalamic paraventricular nucleus, exerts opposing effects on GnRH-I and GnRH-II) expressing neurons, suggesting a role of NPY in the modulation of hypothalamus-pituitary-gonadal (HPG) activity93,94,95. The Pekin ducks (Anas platyrhynchos domestica) faced with food deprivations showed an enhanced activity of the GnIH neurons96.
Further, assuming a common functional basis across seasonal and non-seasonal species, the feeding times could affect the thyroid hormone responsive pathway involving genes coding for the types 2 and 3 deiodinases (dio2, dio3), as reported in the photoperiodic induction of gonadal maturation in seasonally breeding species; e.g., Japanese quail (Coturnix japonica), blackheaded bunting, Emberiza melanocephala95,97,98. In this pathway, the reciprocal switching of dio2 and dio3 gene expressions regulate local T3 concentration, and consequently the GnRH-I and GnRH-II secretions97. In non-seasonal zebra finches, however, the hypothalamic expressions of dio2 and dio3 genes were found not correlated with the TRF-induced reproductive responses25. TRF-induced differential reproductive performance may also lie in differential activation of the hypothalamic GnRH-II and GnIH pathways influencing the social facilitation and reproductive behavior of breeding pairs25,99. The feeding–fasting times can modulate also the GnRH-II secretion, as shown by changes in the hypothalamic GnRH-II-ir and testicular GnIH levels in zebra finches25,100. Mishra et al.25 also found a negative correlation of GnRH-II-ir with the egg laying latency and a positive correlation with the reproductive success (offsprings/brood/pair) in zebra finches subjected to 4-h TRF under the 12 L;12D25.
The dopaminergic pathway also contributes to the reproductive success; in fact, the dopamine (DA) expressing neurons project to the hypothalamic POA (preoptic area) and PVN (paraventricular nucleus) regions. DA activity affects the GnRH synthesis and HPG axis activity downstream, and it influences both sexual behavior (motivation) and parental care101,102,103. Mishra et al.26 found an enhanced reproductive performance in zebra finch pairs which had increased hypothalamic TH-ir (tyrosine hydroxylase, a key enzyme of the DA biosynthesis). Increased hypothalamic th mRNA levels were also found in redheaded buntings (Emberiza bruniceps) exhibiting the photostimulated spring migratory and reproductive phenotypes104.
Indirect effect: Involvement of circadian clock
The circadian clock is intrinsically related to the metabolic homeostasis, as shown by several studies demonstrating alterations in 24-h clock gene oscillations in response to the desynchronization of feeding times with that of the external LD cycle45,105,106,107,108,109,110,111. Such circadian rhythm disruptions lead to the body fattening and hepatic accumulation of lipids, and hence cause dyslipidemia exhibiting abnormal levels of cholesterol and other lipids45,112. There appears a complex interplay of the circadian clock, timings of feeding–fasting and meals, sleep and gut microbiome (Fig. 5, see the perspective section), which may exert influence on circadian rhythms at the cellular and organ levels. This is exhibited in situations where feeding occurs at the wrong time of the day; in other words, at inappropriate intervals relative to the body’s circadian clock113.
A graphical representation of the putative functional connections and integration to achieve homeostasis between the internal circadian clock, wake-sleep cycle, eating pattern and gut microbiome. The bottom panel shows the effect of feeding patterns on health and fitness under the natural and unnatural light-dark environment.
Is feeding–fasting cycle an input to the circadian clock?
A key question is whether recurring 24-h feeding–fasting cycle feedbacks to the internal circadian clocks, and if yes, whether it affects circadian behaviors. To address this, the first line of evidence is from experiments investigating the synchronizing effect of 24-h food presence: food absence (feeding–fasting) cycles on the locomotion rhythm in diurnal birds114,115,116,117,118. Interestingly, both the duration and amount of nocturnal restlessness (Zugunruhe, migratory activity in a caged situation) were reduced when the food availability was restricted only at night but not when it was available only during the daytime in migratory blackheaded buntings, Emberiza melanocephala118. This suggested a hierarchical influence of the environmental time cues: feeding–fasting cycle acts as a synchronizer in the absence of the more dominant LD cycle. However, the feeding–fasting cycle enhances the robustness or amplitude of the mRNA rhythm of clock in rodents33.
A second line of evidence stems from recent studies investigating the impact of temporally disrupted LD environment on clock-controlled behavior and physiology in mammals and birds under both free-living and captive conditions30,45,46,119,120. The dLAN widens the temporal window for feeding times along with increased hunger and appetite sensation, but negatively affects both 24-h activity and sleep patterns. This possibly is the resultant effect of dLAN on the circadian clock gene oscillations, as reported in zebra finches45,46 (Fig. 2g-i). More specifically, there were changes in the peak expression time and/ or amplitude of 24-h rhythms in period 2 (per 2), bmal1 (brain muscle arnt like 1) and reverb b (retinoid-related orphan receptor-beta) genes46 (Fig. 2g-i). Concomitantly, the genes involved in Ca2+-dependent sleep-inducing pathways (camk2, sik3 and nr3a genes) lost a significant 24-h rhythm in their expressions in zebra finches under the dLAN environment46. The caveat, however, is that the circadian clock-dependence of food availability effects fails to account for homeostatic adjustments vis-à-vis behavioral and physiological effects that individuals might undergo when faced with a limited food supply121.
Indirect effects: Epigenetic regulation
Epigenetics refers to heritable changes in gene functions that occur without a change in the DNA sequence122. These changes are less stable, and can be modified in response to the external stimuli. The changes include the chemical modification of DNA and chromatin proteins, which affect the DNA accessibility regulating a wide range of DNA-templated processes, including the expression of candidate genes. Increasing evidences recognize the epigenetic basis of changes in behavioral and physiological phenotypes as a function of changes in the surrounding environment, including food availability8,9,10. Few studies have focused on functional linkage of TRF with the epigenetic regulation of physiology and behavior. For example, a reduced histone deacetylase (HDAC) activity, and hence an enhanced histone H3 acetylation, was found in the hippocampus of rats subjected to TRF conditions18. Mishra et al.27 have reported changes in the expression of genes involved in DNA methylation (dnmts, tets) and histone modification (hat1, hdacs) in the hypothalamus, liver and gonads of zebra finches subjected to a 4-h TRF (see above), although with tissue-specific and sex-dependent expression patterns. Both sexes had similar hypothalamic and hepatic mRNA expression patterns of hat1 and hdac(s), but different dnmt(s) and tet(s) genes expression patterns27. Importantly, the hypothalamic hdac(s) mRNA levels were increased in the TRF condition, irrespective of the food timings (Fig. 3)27.
Perspectives
Ecological significance
Both, the spatial and temporal distributions of food availability are closely coupled to the foraging pattern; species space out their feeding areas or their feeding times during the 24-h day. For example, among species sharing the same community space, some can feed on the available food resource earlier in day than the others123. Similarly, different species inhabiting the same ecological niche can show resource partitioning by using different but closely resembling food resources124. The lion-tailed macaque (Macaca silenus) and Nilgiri langur (Semnopithecus johnii) coexist with their main diet from same trees but consisting of fruits and leaves, respectively124. Likewise, the seasonal abundance of insect food coincides with the energetically demanding egg production and offspring feeding in many bird species125,126,127.
Co-evolution food and feeding patterns
Food
Animals adapt to particular features of food they consume (diet), as a result of a close interplay of their digestive physiology with food environment128. Thus, there is a huge diversity in food and feeding habits as well as in the distribution of feeding times among species inhabiting a shared ecosystem128,129. A significant biological factor for the variations in feeding habits is the gut microbiome, which has the lowest bacterial diversity in carnivores, intermediate in omnivores and highest in herbivores130,131. The microbial diversity adds to the metabolic flexibility of host’s physiology, as it can change with the diet shift. Turnbaugh et al.132 found changes in the phylogenetic composition of microbiome when mice were switched from a plant-based, low-fat diet to a sugar- and fat-rich diet, although changes were ceased after about a week of the diet shift. An adverse effect on gut bacterial diversity along with that on the host physiology was also found in zebra finches feeding at night under the dLAN environment133. More specifically, the dLAN environment altered gut microbiota with pathogenic Proteobacteria abundance, Lactobacillus decline was correlated with negative effects on host’s physiology, and Lactobacillus rhamnosus GG supplement mitigated the adverse metabolic dLAN-induced effects in zebra finches133.
The migratory species may encounter consistent variations in the habitat while enroute as well as vastly different environmental conditions between the breeding and overwintering grounds. They show significant fluctuations in the gut microbiome as per the feeding resource available to them. For example, a divergence in the intestinal microbiota was found in barn swallow (Hirundo rustica) between its migrant and resident subspecies, perhaps because of their different breeding sites134. Interestingly, in migratory birds, the gut microbiota may show similarity within and between species during the migratory travel, which is attributed to the host’s access to the similar food resources and external environment135.
Feeding pattern
There is a close relationship of the appetite in consumers and the food availability. This is best exemplified by the relationship between temporal activity patterns of predators and their preys. The predator and its prey appear locked evolutionarily in shaping their mutual behaviors and life history traits. There is almost universal temporal variation in the feeding over daily136, lunar137, and seasonal138 time scales in predator–prey systems. In a theoretical avian study, Lang et al.139 compared data of predators with songbird prey species, and concluded that the evolution of temporal strategies in predators was to match and exploit temporal patterns in the activity and feeding behavior of their preys.
Phase relationships
Circadian clocks in brain and peripheral (e.g., gut, liver) tissues achieve the phase relationships between when to eat (feeding time) and what to eat (food). There is evidence for the interaction between intestinal clock and the rhythmic abundance of microbial diversity to maintain gut homeostasis140,141. The clocks establish a flexible relationship such that daily activity behavior is synchronized between consumer and food available142. There is also functional interaction between the host’s circadian rhythms, and its eating behavior and gut microbiota143. This relationship can breakdown in the event of change in the natural feeding pattern such as under the dLAN, environment. dLAN-induced desynchronization of circadian rhythms altered gut microbial diversity in zebra finches132. Indeed, the circadian rhythm disruptions cause alterations in microbial communities, and in turn, induce negative effects on host metabolism and energy homeostasis, leading to the metabolic syndrome144.
Concluding remarks
In spite of counter arguments, the accumulated evidence in favor of both circadian rhythm and other hypothalamic pathways are consistent with the overall idea that animals modulate and optimize various physiological processes, particularly those underlying the reproduction in response to resultant metabolic signals emanating from the feeding–fasting periods. This may involve concurrent activation of a number of molecular pathways in the brain and peripheral (e.g., liver and muscle) tissues associated with the regulation of daily sleep, metabolism, sexual motivation (e.g., desire to engage sexually), and reproduction. Importantly, the parents can also adopt to food provisioning in the face of a limited feeding resource; hence, there is quality–quantity trade-off for the reproductive performance, which in the long term may affect the population dynamics of a species.
Figure 5 summarizes the complex interaction relationship that possibly exists between the circadian rhythms, feeding pattern and diet, and gut microbiome in both animals and humans. This can be experimentally shown by experiments in which desynchronization of the circadian rhythms leads to alterations in the abundance and functionality of gut microbiota as well as concurrent deleterious effects on the metabolism and reproduction, and the overall health and reproductive fitness. Therefore, the key to good health lies in crucial meal timings in the appropriate phase relationship with the internal circadian clock, rendering the circadian homeostasis. Thus, using animal model systems, an exciting area for future research is to decipher the linkage between circadian rhythms, feeding times, and gut microbial patterns in relation to disease frameworks in humans.
Data availability
No datasets were generated or analyzed during the current study.
References
Galgani, J. & Ravussin, E. Energy metabolism, fuel selection, and body weight regulation. Int. J. Obes. (Lond.) Suppl. 7, S109–S119 (2008).
Kumar, V., Sharma, A. & Tripathi, V. Physiological effects of food availability times in higher vertebrates. J. Exp. Biol. 225, jeb239004 (2022).
Mishra, I. & Kumar, V. The quantity–quality trade-off: differential effects of daily food availability times on reproductive performance and offspring quality in diurnal zebra finches. J. Exp. Biol. 222, jeb196667 (2019).
Harper, J. M., Leathers, C. W. & Austad, S. N. Does caloric restriction extend life in wild mice? Aging Cell 5, 441–449 (2006).
Ottinger, M. A. et al. Effects of calorie restriction on reproductive and adrenal systems in Japanese quail: are responses similar to mammals, particularly primates? Mech. Ageing Dev. 126, 967–975 (2005).
Hatori, M. et al. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 15, 848–860 (2012).
Renema, R. A. et al. The use of feed restriction for improving reproductive traits in male-line large white Turkey hens: 2. Ovary morphology and laying traits. Poul. Sci. 74, 102–120 (1995).
Bossdorf, O., Richards, C. L. & Pigliucci, M. Epigenetics for ecologists. Ecol Lett. 11, 106–115 (2008).
Jablonka, E. & Lamb, J. R. in Evolutionary Genetics: Concepts and Case Studies (eds. Fox, C. W. & Wolf, J. B.) 252–264 (Oxford University Press, 2006).
Verhoeven, K. J. F., vonHoldt, B. M. & Sork, V. L. Epigenetics in ecology and evolution: what we know and what we need to know. Mol. Ecol. 25, 1631–1638 (2016).
Bohacek, J. & Mansuy, I. M. Molecular insights into transgenerational non-genetic inheritance of acquired behaviours. Nat. Rev. Genet. 16, 641–652 (2015).
Chen, Z., Li, S., Subramaniam, S., Shyy, J. Y. J. & Chien, S. Epigenetic regulation: a new frontier for biomedical engineers. Annu. Rev. Biomed. Eng. 19, 195–219 (2017).
Klemm, S. L., Shipony, Z. & Greenleaf, W. J. Chromatin accessibility and regulatory epigenome. Nat. Rev. Genet. 20, 207–220 (2019).
Sepers, B. et al. Avian ecological epigenetics: pitfalls and promises. J. Ornithol. 160, 1183–1203 (2019).
Dunn, G. A. & Bale, T. L. Maternal high-fat diet promotes body length increases and insulin insensitivity in second-generation mice. Endocrinology 150, 4999–5009 (2009).
Zheng, S. & Pan, Y. X. Histone modifications, not DNA methylation, cause transcriptional repression of p16 (CDKN2A) in the mammary glands of offspring of protein-restricted rats. J. Nutr. Biochem. 22, 567–573 (2010).
Zheng, S., Li, Q., Zhang, Y., Balluff, Z. & Pan, Y. X. Histone deacetylase 3 (HDAC3) participates in the transcriptional repression of the p16INK4a gene in mammary gland of the female rat offspring exposed to an early-life high-fat diet. Epigenetics 7, 183–190 (2012).
Landgrave-Gómez, J. et al. Anticonvulsant effect of time-restricted feeding in a pilocarpine-induced seizure model: metabolic and epigenetic implications. Front. Cell. Neurosci. 10, 7 (2016).
Chaix, A., Zarrinpar, A., Miu, P. & Panda, S. Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab. 20, 991–1005 (2014).
Chaix, A., Lin, T., Le, H. D., Chang, M. W. & Panda, S. Time-restricted feeding prevents obesity and metabolic syndrome in mice lacking a circadian clock. Cell Metab. 29, 303–319 (2019).
Panda, S. Circadian physiology of metabolism. Science 354, 1008–1015 (2016).
BaHammam, A. S. & Pirzada, A. Timing Matters: The interplay between early mealtime, circadian rhythms, gene expression, circadian hormones, and metabolism-a narrative review. Clocks Sleep 5, 507–535 (2023).
Bass, J. Interorgan rhythmicity as a feature of healthful metabolism. Cell Metab. 36, 655–669 (2024).
Ezpeleta, M., Cienfuegos, S., Lin, S., Pavlou, V., Gabel, K., Tussing-Humphreys, L. & Varady, K. A. Time-restricted eating: Watching the clock to treat obesity. Cell Metab. 36, 301–314 (2023).
Mishra, I. et al. Changes in brain peptides associated with reproduction and energy homeostasis: putative roles of gonadotrophin-releasing hormone-II and tyrosine hydroxylase in determining reproductive performance in response to daily food availability times in diurnal zebra finches. J. Neuroendocrinol. 32, jne12825 (2020).
Mishra, I. et al. Developmental effects of daily food availability times on song behavior and neuronal plasticity of song-control system in male zebra finches. Behav. Brain Res. 382, 112497 (2020).
Mishra, I. et al. Changes in DNA methylation and histone modification gene expression in response to daily food times in zebra finches: epigenetic implications. J. Exp. Biol. 223, jeb 217422 (2020).
Falchi, F. et al. The new world atlas of artificial night sky brightness. Sci. Adv. 2, e1600377 (2016).
Gaston, K. J., Davies, T. W., Nedelec, S. L. & Holt, L. A. Impacts of artificial light at night on biological timings. Annu. Rev. Ecol. Evol. Syst. 48, 49–68 (2017).
Navara, K. J. & Nelson, R. J. The dark side of light at night: physiological, epidemiological, and ecological consequences. J. Pineal Res. 43, 215–224 (2007).
McGlade, C. L. O., Capilla-Lasheras, P., Womack, R. J., Helm, B. & Dominoni, D. M. Experimental light at night explain differences in activity onset between urban and forest great tits. Biol. Lett. 19, 20230194 (2023).
Kumar, V. & Gwinner, E. Pinealectomy shortens resynchronisation times of house sparrow (Passer domesticus) circadian rhythms. Naturwissenschaften 92, 419–422 (2005).
Longo, V. D. & Panda, S. Fasting, circadian rhythms, and time-restricted feeding in healthy lifespan. Cell Metab. 23, 1048–1059 (2016).
Kumar, V. Biological Timekeeping: Clocks, Rhythms and Behavior (Springer, 2017).
Daan, S. & Aschoff, J. Circadian rhythms of locomotor activity in captive birds and mammals: Their variations with season and latitude. Oecologia 18, 269–316 (1975).
Gupta, N. J. & Kumar, V. Testes play a role in termination but not in initiation of the spring migration in the night-migratory blackheaded bunting. Anim. Biol. 63, 321–329 (2013).
Aschoff, J. Handbook of Behavioral Neurobiology: Vol. 4: Biological Rhythms 563 (Plenum Press, 1981).
Kumar, V., Gwinner, G. & Van’t Hof, T. J. Circadian rhythms of melatonin in the European starling exposed to different lighting conditions: relationship with locomotor and feeding rhythms. J. Comp. Physiol. A 186, 205–215 (2000).
Kumar, V., Van’t Hof, T. J. & Gwinner, E. Circadian behavioral and melatonin rhythms in the European starling under light-dark cycles with steadily changing periods: Evidence for close mutual coupling. Horm. Behav. 52, 409–416 (2007).
Kumar, V., Kumar, B. S. & Singh, B. P. Photostimulation of blackheaded bunting: Subjective interpretation of day and night depends upon both photophase contrast and light intensity. Physiol. Behav. 51, 1213–1217 (1992).
Singh, J., Rani, S. & Kumar, V. Functional similarity in relation to the external environment between circadian behavioral and melatonin rhythms in the subtropical Indian weaverbird. Horm. Behav. 61, 527–534 (2012).
Taufique, S. K. T. & Kumar, V. Differential activation and tyrosine hydroxylase distribution in the hippocampal, pallial and midbrain brain regions in response to cognitive performance in Indian house crows exposed to abrupt light environment. Behav. Brain Res. 314, 21–29 (2016).
Jha, N. A. & Kumar, V. Effect of no-night light environment on behavior, learning performance and personality in zebra finches. Anim. Behav. 132, 29–47 (2017).
Taufique, S. K. T., Prabhat, A. & Kumar, V. Illuminated night alters hippocampal gene expressions and induces depressive‐like responses in diurnal corvids. Eur J Neurosci. 48, 3005–3018 (2018).
Batra, T., Malik, I. & Kumar, V. Illuminated night alters behavior and negatively affects physiology and metabolism in diurnal zebra finches. Environ. Pollut. 254, 112916 (2019).
Batra, T., Malik, I., Prabhat, A., Bhardwaj, S. K. & Kumar, V. Sleep in unnatural times: Illuminated night negatively affects sleep and associated hypothalamic gene expressions in diurnal zebra finches. Proc. Roy. Soc. B 287, 20192952 (2020).
Prabhat, A., Batra, T. & Kumar, V. Effects of timed food availability on reproduction and metabolism in zebra finches: Molecular insights into homeostatic adaptation to food-restriction in diurnal vertebrates. Horm. Behav. 125, 104820 (2020).
Batra, T., Buniyaadi, A. & Kumar, V. Daytime restriction of feeding prevents illuminated night-induced impairment of metabolism and sleep in diurnal zebra finches. Physiol. Behav. 253, 113866 (2022).
Dominoni, D. M. et al. Integrated molecular and behavioral data reveal deep circadian disruption in response to artificial light at night in male Great tits (Parus major). Sci. Rep. 12, 1553 (2022).
Bus, J. D. et al. Circadian rhythms and diurnal patterns in the feed intake behavior of growing-finishing pigs. Sci. Rep. 13, 16021 (2023).
Cross, A. J., Brown-Brandl, T. M., Keel, B. N., Cassady, J. P. & Rohrer, G. A. Feeding behavior of grow-finish swine and the impacts of heat stress. Transl. Anim. Sci. 4, 1–7 (2020).
Rani, S., Singh, S., Malik, S. & Kumar, V. in Biological Timekeeping: Clocks, Rhythms and Behavior (ed. Kumar, V.) 625–642 (Springer Nature (Springer India), 2017).
Scheer, F. A., Hilton, M. F., Mantzoros, C. S. & Shea, S. A. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc. Natl Acad. Sci. USA 106, 4453–4458 (2009).
Duffy, J. F. & Dijk, D.-J. Getting through to circadian oscillators: why use constant routines? J. Biol. Rhythms. 17, 4–13 (2002).
Wehrens, S. M. T. et al. Meal timing regulates the human circadian system. Curr Biol. 27, 1768–1775 (2017).
O’Reardon, J. P. et al. Circadian eating and sleeping patterns in the night eating syndrome. Obes Res. 12, 1789–1796 (2004).
Szosland, D. Shift work and metabolic syndrome, diabetes mellitus and ischemic heart disease. Int. J. Occu. Med. Environ. Health 23, 287 (2010).
Kumar, V., Singh, S., Misra, M. & Malik, S. Effects of duration and time of food availability on photoperiodic responses in the migratory male blackheaded bunting Emberiza melanocephala. J. Exp. Biol. 204, 2843–2848 (2001).
Bhardwaj, S. K. The effect of duration and time of food availability on the photoperiodic response in the male house sparrow, Passer domesticus. Reprod. Nutrition Dev. 44, 29–35 (2004).
Cappuccio, F. P. et al. Meta-analysis of short sleep duration and obesity in children and adults. Sleep 31, 619–626 (2008).
Spiegel, K., Tasali, E., Leproult, R. & Van Cauter, E. Effects of poor and short sleep on glucose metabolism and obesity risk. Nat. Rev. Endocr. 5, 253–261 (2009).
Yasumoto, Y. et al. Short-term feeding at the wrong time is sufficient to desynchronize peripheral clocks and induce obesity with hyperphagia, physical inactivity and metabolic disorders in mice. Metabolism 65, 714–727 (2016).
Prabhat, A., Buniyaadi, A., Bhardwaj, S. K. & Kumar, V. Differential effects of continuous and intermittent daytime food deprivation periods on metabolism and reproductive performance in diurnal zebra finches. Horm. Behav. 152, 105353 (2023).
Fonken, L. K. et al. Light at night increases body mass by shifting the time of food intake. Proc. Natl Acad. Sci. 107, 18664–18669 (2010).
Mehlem, A., Hagberg, C. E., Muhl, L., Eriksson, U. & Falkevall, A. Imaging of neutral lipids by oil red O for analyzing the metabolic status in health and disease. Nat. Protoc. 8, 1149 (2013).
Opperhuizen, A. L. et al. Light at night acutely impairs glucose tolerance in a time-, intensity-and wavelength-dependent manner in rats. Diabetologia 60, 1333–1343 (2017).
Jensen-Urstad, A. P. & Semenkovich, C. F. Fatty acid synthase and liver triglyceride metabolism: housekeeper or messenger? Biochem. Biophys. Acta Mol. Cell Biol. Lipids 1821, 747–753 (2012).
Jieun, L., Kyoungho, L., Seungyoun, J. & Park, Y. J. Associations of meal timing and sleep duration with incidence of obesity: a prospective cohort study. J. Nutr. health and aging 28, 100220 (2024).
Duan, D., Gu, C., Polotsky, V. Y., Jun, J. C. & Pham, L. V. Effects of dinner timing on sleep stage distribution and EEG power spectrum in healthy volunteers. Nat. Sci. Sleep 13, 601–612 (2021).
Buniyaadi, A., Prabhat, A., Bhardwaj, S. K. & Kumar, V. Night melatonin levels affect cognition in diurnal animals: molecular insights from a corvid exposed to an illuminated night environment. Environ. Pollut. 308, 119618 (2022).
Weljie, A. M. et al. Oxalic acid and diacylglycerol 36: 3 are cross-species markers of sleep debt. Proc. Natl Acad. Sci. USA 112, 2569–2574 (2015).
Onagbesan, O. M. et al. Effects of genotype and feed allowance on plasma luteinizing hormones, follicle-stimulating hormones, progesterone, estradiol levels, follicle differentiation, and egg production rates of broiler breeder hens. Poult. Sci. 85, 1245–1258 (2006).
Valle, S., Carpentier, E., Vu, B., Tsutsui, K. & Deviche, P. Food restriction negatively affects multiple levels of the reproductive axis in male house finches, Haemorhous mexicanus. J. Exp. Biol. 218, 2694–2704 (2015).
Lynn, S. E., Stamplis, T. B., Barrington, W. T., Weida, N. & Hudak, C. A. Food, stress, and reproduction: short-term fasting alters endocrine physiology and reproductive behavior in the zebra finch. Horm. Behav. 58, 214–222 (2010).
Meijer, T. The effect of a period of food restriction on gonad size and moult of male and female starlings Sturnus vulgaris under constant photoperiod. Ibis 133, 80–84 (1991).
Davies, S. et al. Food availability, energetic constraints and reproductive development in a wild seasonally breeding songbird. Funct. Ecol. 29, 1421–1434 (2015).
Bradshaw, C. J. A., & McMahon, C. R. in Encyclopedia of Ecology (eds. Jorgensen, S. E. & Fath, B. D.) 1535–1543 (Elsevier Inc., 2008).
Reynolds, S. J., Schoech, S. J. & Bowman, R. Nutritional quality of prebreeding diet influences breeding performance of the Florida scrub jay. Oecologia 134, 308–316 (2003).
Timper, K. & Brüning, J. C. Hypothalamic circuits regulating appetite and energy homeostasis: pathways to obesity. Dis Model Mech. 10, 679–689 (2017).
Maurer, B. A. in Avian Energetics and Nutritional Ecology (ed. Carey, C.) 250–279 (Springer, 1996).
Hetherington, A. W. & Ranson, S. W. Hypothalamic lesions and adiposity in the rat. Anat. Rec. 78, 149–172 (1940).
Anand, B. K. & Brobeck, J. R. Localization of a “feeding center” in the hypothalamis of the rat. Proc. Soc. Exp. Biol. Med. 77, 323–324 (1951).
Smith, P. M. & Ferguson, A. V. Neurophysiology of hunger and satiety. Dev. Disabil. Res. Rev. 14, 96–104 (2008).
Coll, A. P., Farooqi, I. S. & O’Rahilly, S. The hormonal control of food intake. Cell 129, P251–P262 (2007).
Coleen, R. et al. The role of the human hypothalamus in food intake networks: an MRI perspective. Front. Nutr. 8, 760914 (2022).
Singh, O. et al. Cocaine- and amphetamine-regulated transcript peptide (CART) in the brain of zebra finch, Taeniopygia guttata: organization, interaction with neuropeptide Y, and response to changes in energy status. J. Comp. Neurol. 524, 3014–3041 (2016).
Huang, Y., Lin, X. & Lin, S. Neuropeptide Y and metabolism syndrome: An update on perspectives of clinical therapeutic intervention strategies. Front. Cell Dev. Biol. 9, 695623 (2021).
Kuhar, M. J., Adams, L. D., Hunter, R. G., Vechia, S. D. & Smith, Y. CART peptides. Regul Pept. 89, 1–6 (2000).
Singh, D., Kumari, Y., Rastogi, A., Rani, S. & Kumar, V. Neuropeptide Y mRNA and peptide in the night-migratory redheaded bunting brain. Cell Tiss. Res. 354, 551–562 (2013).
Ahmadian-Moghadam, H., Sadat-Shirazi, M. S. & Zarrindast, M. R. Cocaine- and amphetamine-regulated transcript (CART): A multifaceted neuropeptide. Peptides 110, 56–77 (2018).
Crown, A., Clifton, D. K. & Steiner, R. A. Neuropeptide signaling in the integration of metabolism and reproduction. Neuroendocrinology 86, 175–182 (2007).
Garcia-Garcia, R. M. Integrative control of energy balance and reproduction in females. ISRN Vet Sci. 26, 121389 (2012).
Ubuka, T. et al. Gonadotropin-inhibitory hormone neurons interact directly with gonadotropin-releasing hormone-I and -II neurons in European starling brain. Endocrinology 149, 268–278 (2008).
McConn, B. et al. Gonadotropin-inhibitory hormone-stimulation of food intake is mediated by hypothalamic effects in chicks. Neuropeptides 48, 327–334 (2014).
Rastogi, A., Rani, S. & Kumar, V. Seasonal plasticity in the peptide neuronal systems: potential roles of gonadotrophin-releasing hormone, gonadotrophin-inhibiting hormone, neuropeptide Y and vasoactive intestinal peptide in the regulation of the reproductive axis in subtropical Indian weaverbirds. J. Neuroendocrinol. 27, 357–369 (2015).
Fraley, G. S. et al. Distribution and sequence of gonadotropin-inhibitory hormone and its potential role as a molecular link between feeding and reproductive systems in the pekin duck (Anas platyrhynchos domestica). Gen. Comp. Endocrinol. 184, 103–110 (2013).
Nakane, Y. & Yoshimura, T. Universality and diversity in the signal transduction pathway that regulates seasonal reproduction in vertebrates. Front. Neurosci. 8, 115 (2014).
Kumar, V. et al. Avian photoreceptors and their role in the regulation of daily and seasonal physiology. Gen. Comp. Endocrinol. 220, 13–22 (2015).
Stevenson, T. J. et al. Effects of social cues on GnRH-I, GnRH-II, and reproductive physiology in female house sparrows (Passer domesticus). Gen. Comp. Endocrinol. 156, 385–394 (2008).
Lynn, S. E., Perfito, N., Guardado, D. & Bentley, G. E. Food, stress and circulating testosterone: cue integration by the testes, not the brain, in male zebra finches (Taeniopygia guttata). Gen. Comp. Endocrinol. 215, 1–9 (2015).
Clarkson, J. & Herbison, A. E. Dual phenotype kisspeptin-dopamine neurons of the rostral periventricular area of the third ventricle project to gonadotrophin-releasing hormone neurons. J. Neuroendocrinol. 23, 293–301 (2011).
Klingerman, C. M. et al. Food restriction-induced changes in gonadotropin-inhibiting hormone cells are associated with changes in sexual motivation and food hoarding, but not sexual performance and food intake. Front. Endocrinol. 2, 1–15 (2011).
Hewlett, S. E. et al. Hypothalamic vasotocin and tyrosine hydroxylase levels following maternal care and selection for low mortality in laying hens. BMC Vet. Res. 10, 167 (2014).
Sharma, A. et al. Difference in control between spring and autumn migration in birds: insight from seasonal changes in hypothalamic gene expression in captive buntings. Proc. R. Soc. B 285, 20181531 (2018).
Damiola, F. et al. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 14, 2950–2961 (2000).
Turek, F. W. et al. Obesity and metabolic syndrome in circadian Clock mutant mice. Science. 308, 1043–1045 (2005).
Lamia, K. A., Storch, K. F. & Weitz, C. J. Physiological significance of a peripheral tissue circadian clock. Proc. Natl Acad. Sci. 105, 15172–15177 (2008).
Yang, S. et al. The role of mPer2 clock gene in glucocorticoid and feeding rhythms. Endocrinol. 150, 2153–2160 (2009).
Marcheva, B. et al. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature 466, 627–631 (2010).
Shimba, S. et al. Deficient of a clock gene, brain and muscle Arnt-like protein-1 (BMAL1), induces dyslipidemia and ectopic fat formation. PLoS ONE 6, e25231 (2011).
Cho, H. et al. Regulation of circadian behavior and metabolism by REV-ERB-α and REV-ERB-β. Nature 485, 123–127 (2012).
Rudic, R. D. et al. BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis. PLoS Biol. 2, e377 (2004).
Jakubowicz, D., Rosenblum, R. C., Wainstein, J. & Twito, O. Influence of Fasting until noon (extended Postabsorptive State) on clock gene mRNA expression and regulation of body weight and glucose metabolism. Int. J. Mol. Sci. 24, 7154 (2023).
Hahn, T. P. Integration of photoperiodic and food cues to time changes in reproductive physiology by an opportunistic breeder, the red crossbill, Loxia curvirostra (Aves: Carduelinae). J. Exp. Zool. 272, 213–226 (1995).
Hau, M. & Gwinner, E. Food as a circadian Zeitgeber for house sparrows: the effect of different food access durations. J. Biol. Rhythms 11, 196–207 (1996).
Perfito, N., Kwong, J. M. Y., Bentley, G. E. & Hau, M. Cue hierarchies and testicular development: Is food a more potent stimulus than day length in an opportunistic breeder (Taeniopygia guttata)? Horm. Behav. 53, 567–572 (2008).
Rani, S., Singh, S., Malik, S., Singh, J. & Kumar, V. Synchronization of Indian weaverbird circadian rhythms to food and light zeitgebers: role of pineal. Chronobiol. Int. 26, 653–665 (2009).
Singh, J., Rastogi, A., Rani, S. & Kumar, V. Food availability affects circadian clock-controlled activity and zugunruhe in the night migratory male blackheaded bunting (Emberiza melanocephala). Chronobiol. Int. 29, 15–25 (2012).
Raap, T., Pinxten, R. & Eens, M. Light pollution disrupts sleep in free-living animals. Sci. Rep. 5, 13557 (2015).
Stenvers, D. J. et al. Dim light at night disturbs the daily sleep-wake cycle in the rat. Sci. Rep. 6, 35662 (2016).
Dall, S. R. X. & Witter, M. S. Feeding interruptions, diurnal mass changes and daily routines of behavior in zebra finches. Anim. Behav. 55, 715–725 (1998).
Goldberg, A. D., Allis, C. D. & Bernstein, E. Epigenetics: a landscape takes shape. Cell 128, 635–638 (2007).
Terborgh, J. Five New World Primates: A Study in Comparative Ecology (Princeton University Press, 1983).
Sushma, H. S. & Singh, M. Resource partitioning and interspecific interactions among sympatric rain forest arboreal mammals of the Western Ghats, India. Behav. Ecol. 17, 479–490 (2006).
Nagar, R. G. & vanNoordwijk, A. J. Proximate and ultimate aspects of phenotypic plasticity in timing of great tit breeding in a heterogeneous environment. Am. Nat. 146, 454–474 (1995).
VanNoordwijk, A. J., McCleery, R. H. & Perrins, C. M. Selection for the timing of great tit breeding in relation to caterpillar growth and temperature. J. Anim. Ecol. 64, 451–458 (1995).
Hahn, T. P., Pereyra, M. E., Katti, M., Ward, G. M. & Macdougall-Shackleton, S. A. in Functional Avian Endocrinology (ed. Dawson, A. & Sharp, P. J.) 167–180 (Narosa Publishing House, 2005).
Karasov, W. H., & Martínez del-Rio, C. Physiological Ecology: How Animals Process Energy, Nutrients, and Toxins (Princeton Univ. Press, 2007)
Karasov, W. H., Martínez del-Rio, C. & Vidal, E. C. Ecological physiology of diet and digestive system. Annu. Rev. Physiol. 73, 69–93 (2011).
Ley, R. E. et al. Evolution of mammals and their gut microbiomes. Science 320, 1647–1651 (2008).
Ley, R. E., Lozupone, C. A., Hamady, M., Knight, R. & Gordon, J. I. Worlds within worlds: Evolution of the vertebrate gut microbiota. Nat. Rev. Microbiol. 6, 776–778 (2008).
Turnbaugh, P. J. et al. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci. Transl. Med. 1, 1–10 (2009).
Malik, I., Batra, T., Das, S. & Kumar, V. Light at night affects gut microbial community and negatively impacts host physiology in diurnal animals: Evidence from captive zebra finches. Microbiol. Res. 241, 126597 (2020).
Turjeman, S. et al. Migration, pathogens and the avian microbiome: a comparative study in sympatric migrants and residents. Mol. Ecol. 29, 4706–4720 (2020).
Lewis, W. B., Moore, F. R. & Wang, S. Changes in gut microbiota of migratory passerines during stopover after crossing an ecological barrier. AUK 134, 137–145 (2017).
Metcalfe, N. B. & Ure, S. E. Diurnal variation in flight performance and hence potential predation risk in small birds. Proc. R. Soc. B 261, 395–400 (1995).
Prugh, L. R. & Golden, C. D. Does moonlight increase predation risk? Meta‐analysis reveals divergent responses of nocturnal mammals to lunar cycles. J. Anim. Eco. 83, 504–514 (2014).
Sperry, J. H., Peak, R. G., Cimprich, D. A. & Weatherhead, P. J. Snake activity affects seasonal variation in nest predation risk for birds. J. Avian Biol. 39, 379–383 (2008).
Lang, S. D. J., Mann, R. P. & Farine, D. R. Temporal activity patterns of predators and prey across broad geographic scales. Behav. Ecol. 30, 172–180 (2019).
Henao-Mejia, J., Strowig, T. & Flavell, R. A. Microbiota keep the intestinal clock ticking. Cell 153, 741–743 (2013).
Heddes, M. et al. The intestinal clock drives the microbiome to maintain gastrointestinal homeostasis. Nat Commun 13, 6068 (2022).
Botts, R. T. et al. Circadian activity patterns of mammalian predators and prey in Costa Rica. J. Mammal. 101, 1313–1331 (2020).
Kaczmarek, J. L., Thompson, S. V. & Holscher, H. D. Complex interactions of circadian rhythms, eating behaviors, and the gastrointestinal microbiota and their potential impact on health. Nutr Rev. 75, 673–682 (2017).
Bishehsari, F., Voigt, R. M. & Keshavarzian, A. Circadian rhythms and the gut microbiota: from the metabolic syndrome to cancer. Nat. Rev. Endocrinol. 12, 731–739 (2020).
Acknowledgements
V.T. gratefully acknowledges the encouragement for his research studies received from the Principal, Dyal Singh College, University of Delhi, New Delhi. V.K. is supported by the Indian National Science Academy, New Delhi, through an award of the Senior Scientist position.
Author information
Authors and Affiliations
Contributions
V.K. wrote the manuscript, V.T. and S.K.B. prepared figures 1-5, VT, S.K.B. reviewed the manuscript, V.K. revised the manuscript. V.T. and S.K.B. reviewed the revised draft, V.K. produced the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Tripathi, V., Bhardwaj, S.K. & Kumar, V. Ecology of timekeeping: feeding times effect clock-controlled behavior, metabolism and reproduction in diurnal vertebrates. npj Biol Timing Sleep 2, 8 (2025). https://doi.org/10.1038/s44323-025-00022-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s44323-025-00022-8
This article is cited by
-
Saving from the dark side of light at night: differential effects of complete darkness in the first and second half of dimly illuminated nights on sleep and metabolism
Journal of Comparative Physiology A (2025)