Abstract
Most aspects of physiology and behaviour fluctuate every 24 h in mammals. These circadian rhythms are orchestrated by an autonomous central clock located in the suprachiasmatic nuclei that coordinates the timing of cellular clocks in tissues throughout the body. The critical role of this circadian system is emphasized by increasing evidence associating disruption of circadian rhythms with diverse pathologies. Accordingly, mounting evidence suggests a bidirectional relationship where disruption of rhythms by circadian misalignment may contribute to liver diseases while liver diseases alter the central clock and circadian rhythms in other tissues. Therefore, liver pathophysiology may broadly impact the circadian system and may provide a mechanistic framework for understanding and targeting metabolic diseases and adjust metabolic setpoints.
Similar content being viewed by others
The mammalian liver circadian clock
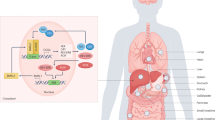
The mammalian circadian system is a network of cellular clocks that coordinates the timing of internal physiological processes in anticipation of daily recuring environmental changes. Nearly every organ and cell in the body harbours a circadian clock. Together, these clocks are organised to produce coherent, ~24-h tissue-level oscillations. In mammals, the central circadian pacemaker is localised in the bilateral suprachiasmatic nuclei (SCN) of the hypothalamus and receives input about environmental light directly from the retina. The SCN clock entrains to the light-dark cycle and then coordinates the timing, or phases, of tissue clocks located throughout the brain and body1,2. Importantly, while the molecular building blocks of the clock are largely preserved across tissues, each tissue clock entrains with a different temporal relationship to the light-dark cycle3,4. The putative value of this circadian organisation is that it temporally partitions behaviour and tissue-specific gene expression and biochemistry so that internal processes anticipate predictable changes in the environment, such as food availability and predation, caused by Earth’s rotation on its axis5 (Fig. 1).
A circadian pacemaker in the hypothalamic suprachiasmatic nuclei (SCN) is reset by the external light-dark cycle. Through temporal regulation of behavioural functions such as the sleep-wake cycle or feeding-fasting rhythms, endocrine outputs and the autonomic nervous system (ANS), the SCN clock controls the timing of cellular clocks in hepatocytes (and other central and peripheral tissues). At the molecular level, these clocks are comprised of interlocked transcriptional-translational feedback loops (TTFLs) of clock genes and proteins characterised by marked 24-h rhythms in transcriptional activity and protein abundance. In the liver, local clock rhythms and internal (e.g., hormones) and external circadian inputs (e.g., meal schedules) are integrated to generate metabolic clock outputs such as glycogen storage/release, bile acid and triglyceride biosynthesis.
The liver is arguably the most well-studied peripheral circadian clock in mammals. It responds dynamically to feeding and fasting cycles and, when nutrients are abundant, it stores energy as glycogen, triglycerides and proteins. Conversely, during periods of fasting or increased energy demand, the liver mobilises energy reserves. Most liver functions have 24-h rhythms to anticipate periods of energy supply and demands. In addition to regulating daily cycles of nutrients, the liver also rhythmically metabolises bile acids, amino acids, toxins and drugs (for reviews see refs. 6,7). Circadian rhythms in liver function are regulated in part by the molecular circadian timekeeping mechanism. The molecular clock in mammals is based on a set of transcriptional-translational negative feedback loop. The circadian protein BMAL1 heterodimerizes with CLOCK (or NPAS2 in some tissues) to drive the transcription of Period (Per1, 2, 3) and Cryptochrome (Cry1, 2) genes. As PER and CRY proteins are translated, they dimerise, translocate into the nucleus and feedback on BMAL1/CLOCK to inhibit their own transcription. This cycle takes approximately 24 h to complete and thus sets the pace of cellular circadian clocks. An additional accessory feedback loops contribute to cellular timekeeping. For example, the BMAL1/CLOCK regulated nuclear receptors ROR (α, γ, β) and REV-ERB (α, β) activate and inhibit the expression of Bmal1 and Clock, respectively, and contribute to fine tune the time keeping system8 (Fig. 1).
Meal timing also regulates circadian rhythms in liver gene expression and function. Consequently, perturbing the daily rhythm of food intake has a strong impact on the organisation of the rhythmic liver clock and physiology9,10,11,12,13,14. When feeding is restricted to the inactive phase, the phases of circadian clocks in peripheral tissues involved in energy metabolism, including the liver, white adipose tissue (WAT), and gut, entrain to feeding time independently of the central circadian clock and other non-metabolic tissues clocks15,16. This results in, both, internal (liver, gut etc. vs. SCN) and external circadian misalignment (liver, gut vs. light-dark cycle)17.
While mechanisms are still not entirely clear, food-borne metabolites and postprandial hormone signals such as insulin, oxyntomodulin, glucagon-like peptide 1 and leptin can act as “zeitgebers” (German word for “time giver”) for circadian clocks in peripheral organs and parts of the brain18,19,20,21. Other factors more prevalent during fasting phases (e.g., during sleep), such as fatty acids or glucagon, may act as pre-feeding signals to reset peripheral clocks22,23. Additionally, feeding is associated with an increase in body movements that contribute to the rhythmic body temperature15,24,25 that, in turn, may act as a synchroniser of the peripheral circadian clocks26,27,28,29 (Fig. 1).
In sum, ~24-h rhythms of liver functions are controlled by both the molecular circadian timekeeping mechanism in hepatocytes and by systemic factors, particularly those related to food intake. Thus, the liver is perfectly poised to anticipate predictable changes in nutrient availability as well as respond to unpredictable metabolic demands.
Disruption of rhythmic liver function is associated with metabolic dysfunction-associated steatotic liver disease (MASLD)
Metabolic dysfunction-associated steatotic liver disease (MASLD) is defined by an excessive accumulation of lipid in the liver in the presence of at least one cardiometabolic risk factor. MASLD comprises isolated liver steatosis (metabolic dysfunction-associated steatotic liver (MASL)), steatohepatitis (MASH), as well as fibrosis and cirrhosis. The new definition thus highlights the metabolic roots of the disease and its tight association with type 2 diabetes (T2D), obesity and other cardiometabolic risk factors30,31. MASLD is the most common chronic liver disease and its prevalence is projected to increase even further. At least one in four patients with MASLD has MASH, which is the second leading cause of liver disease in adults scheduled for liver transplantation in the USA32. In patients with obesity and T2D, prevalence of MASLD increase two- to fourfold, depending on age and comorbidities33,34.
Progression of liver damage in MASLD is extremely variable. Whereas simple steatosis reflects non-progressive dysfunctional metabolism, MASH is a chronic liver disease that may progress undiagnosed for years, before eventually emerging as liver failure and hepatocellular carcinoma (HCC). The earliest event initiating MASLD is absolute or relative calorie excess, as confirmed by the link between MASLD and obesity. Limited physical activity or sedentary behaviours are complementary aspects of calorie imbalance, irrespective of body mass index35. As triglyceride synthesis outpaces the capacity for VLDL synthesis and export, triglycerides accumulate within hepatocytes, resulting in steatosis and eventually lipotoxicity36. Additional increased de novo lipogenesis from carbohydrates, specifically fructose, may produce similar lipotoxic effects. Moreover, consumption of sugar-sweetened drinks containing either fructose or sucrose, which is converted to fructose and glucose in the gut, may be more potent than dietary lipids in promoting MASH37,38. Uncontrolled and incomplete lipid oxidation, oxidative stress and activation of the unfolded protein response are two well characterised pathways that promote cell death in MASH. These multiple insults synergistically drive the development and progression of MASLD, particularly in genetically predisposed people.
Increasing evidence suggests an association between MASLD and the perturbation of the daily rhythms in liver physiology, also called circadian disruption. Liver physiology has been studied in rodent models with circadian disruption including light at night39,40, forced work during the resting phase mimicking shift work41, chronic shifts of the light-dark cycle42,43,44, and genetic disruption of molecular clock function in hepatocytes43,45,46. These animal studies demonstrated that circadian disruption causes fatty liver and MASLD. However, recent studies suggest a more contrasted conclusion. In contrast to hepatocyte-specific circadian clock disruption, disabling the molecular clock in the entire animal47,48,49 or in the intestinal epithelium50 protects mice from MASLD and MASH. While the mechanism is not clear, it likely involves perturbations of circadian clock-dependent endocrine regulation and/or nutrient storage that decrease lipid storage and steatosis in the liver and, consequently, the associated fibrosis and inflammation.
In humans, clinical data are still lacking that document a tight link between disruption of liver circadian rhythms and MASLD/MASH. Recent studies of liver biopsies collected at different times of the daily cycle showed that rhythmic gene expression, including circadian clock gene expression, is disrupted in patients with HCC51. Moreover, increased liver fibrosis severity in human was associated with a decreased expression of circadian clock genes48. Another recent report shows that while livers from MASH patients do not have altered expression of circadian clock genes, the time-of-day-dependent expression of hundreds of transcripts involved in cell-to-cell communication, intracellular signalling and energy metabolism were altered52. Amino acid and lipid metabolome rhythms were also altered suggesting that MASH modulates output rhythms. Similarly, circadian disruption caused by chronic jet lag in a “humanised liver” mouse model in which mouse hepatocytes were partially replaced by human hepatocytes led to MASH and HCC with characteristics similar to the human diseases44. Altogether, these studies suggest that perturbation of rhythmic liver physiology is associated with the development of MASLD.
The relationship between circadian disruption and MASLD is further supported by studies of MASLD in shift workers. One study of >8000 shift workers showed no association with MASLD53. However, a more recent study of >6000 rotating and night shift workers in China showed that longer duration of shift work and extended nighttime work hours increased the risk of MASLD by 23%54. In a comparable study of male shift workers, night shift work was associated with elevated alanine transaminase levels, which is a marker of hepatic pathology55. In a large-scale, retrospective analysis of UK Biobank data, night shift work was associated with MASLD, and the risk increased as years of night shift work increased56. Moreover, irregular schedules of shift work and extreme late chronotype were also associated with elevated liver steatosis57. These results are summarised in Table 1. Interestingly, there is an association between the risk of HCC (and other types of cancer, even in the absence of cirrhosis) and geographical location in a time zone58,59. Those in the Western portion of a time zone have greater HCC risk, possibly because they experience more circadian disruption caused by delayed exposure to sunlight.
Another highly prevalent condition with chronodisruptive potential and an impact on liver steatosis is sleep apnoea. Obstructive sleep apnoea (OSA) is characterised by recurring obstruction of the upper airways during sleep. This results in sub-optimal ventilation and intermittent hypoxia which promotes arousal and reduces overall sleep quality60 and alters circadian activity rhythms61. Mounting evidence suggests OSA and chronic intermittent hypoxia (CIH) are risk factors for MASLD62,63,64. In MASLD patients with OSA, CIH triggers an increase in oxidative stress and generation of reactive oxygen species, release of pro-inflammatory cytokines, and induction of systemic inflammation, which further exacerbate liver steatosis and inflammation65. In addition, OSA in a mouse model is associated with tissue-specific transcriptomic changes in circadian rhythmicity and mean 24-h gene expression levels66. Moreover, hypoxic events themselves act as synchronising stimuli for some tissue clocks. Such tissue-specific responses to OSA-associated hypoxic intervals may provoke circadian tissue clock misalignment (or internal desynchrony) reminiscent of what is observed in animal models of shift work67.
These observations support the hypothesis that high amplitude circadian rhythms protect against the development of liver disease. Accordingly, restoring rhythmic feeding in animals during high-fat (HFD) obesogenic regimen with time-restricted feeding68,69 or synchronising the clock using small molecules targeting the ROR/REV-ERB pathway improves MASLD in mice70,71,72. However, in clinical trials, time-restricted feeding was no more effective than caloric restriction in treating obesity and MASLD73,74,75,76.
MASLD and cirrhosis are related to disrupted circadian physiology and sleep
Research on obesity and MASLD has shown that there are reciprocal connections between metabolic liver disease and circadian physiology. Chronic consumption of HFD, which leads to MASLD in mice77, alters circadian physiology and global rhythmic metabolism in mice. HFD consumption disrupts daily rhythms of meal intake and the associated humoral signals, as well as rhythmic gene expression in peripheral tissues including the liver in male mice69,78,79,80,81. During HFD feeding, mice increase their food intake during the light phase, which is the resting phase for nocturnal rodents during which they normally do not eat79. A parallel observation was made in mice carrying a mutation in the circadian Clock gene, which also promotes overweight and obesity82. Genetic animal models of obesity including the mouse Ob and Db mutations of Leptin and its receptor, respectively, also have disrupted eating rhythms83,84,85. The disruption of the eating rhythm by HFD consumption is reversible86 and dependent on sex and strain since female C57BL/6J mice or mouse strains that are resistant to HFD-induced obesity do not have disrupted eating rhythms during HFD feeding87,88.
The SCN circadian pacemaker and circadian entrainment are also affected by HFD consumption in mice. The circadian period of locomotor activity, a direct output of the activity of the SCN clock, is increased during HFD feeding79. Moreover, the response of circadian activity rhythms to a light pulse, a property of the SCN clock, was also altered89. However, these effects are not specific to obesity and metabolic syndrome. Choline-deficient diet, which rapidly induces liver fibrosis with minimum impact on body weight90, causes wide-spread changes liver transcriptome diurnal rhythms including genes associated with lipid metabolism and inflammatory processes91,92. It also advances the phases of the liver and kidney circadian clocks, suggesting an impact on the global circadian physiology91,92.
In humans, the impact of liver diseases on circadian behaviour has been mainly studied in patients with cirrhosis to identify aetiological strategies to manage the sleep-wake disturbances that are common in these patients93 (Fig. 2). Delayed sleep and wake timing, daytime sleepiness, increased sleep latency and interrupted night sleep are more common in patients with cirrhosis than in patients with other chronic diseases (e.g., renal failure requiring dialysis)94,95,96,97. While sleep-wake abnormalities in patients with cirrhosis have traditionally been ascribed to hepatic encephalopathy, they have recently been linked to excessive daytime sleepiness98 and, in its more severe forms, sleep–wake inversion99. Further, the hepatic metabolism of hypnotic drugs is disturbed in cirrhotic patients100 and patients are extremely sensitive to these medications. Therefore, special care must be taken when treating their sleep disturbances. However, the increase in daytime sleepiness also impairs night sleep and the sleep–wake cycle, making difficult to separate in patients the influence of the increased daytime sleep and the perturbed nighttime sleep. Homoeostatic101,102 and circadian abnormalities in sleep–wake regulation have been associated with liver cirrhosis. The latter includes phase delays in the daily rhythms of melatonin103,104 and cortisol105, impaired melatonin responses to light103 and impaired melatonin catabolism106. There is also anecdotal evidence that liver transplantation can reverse sleep disturbances107, suggesting a direct effect of the cirrhotic liver on circadian behaviour. There are other, largely unexplored mechanisms through which cirrhosis, and especially decompensated cirrhosis, might affect the circadian timing system, including endocrine108 and metabolic109 disruptions as well as sympathetic denervation110 potentially affecting the communication with the SCN, abnormal temperature regulation, reduced muscle mass and ability to exercise, and aberrant meal timing (i.e., evening snacking) (reviewed in ref. 7).
Circadian disruption is a risk factor for the development of fatty liver disease. It can arise from external factors such as light at night or shift work-associated alterations in sleep-wake cycles or internal factors such as sleep apnoea, clock gene modifications or metabolic dysfunction. Through alterations in oxidative stress, cytokine release and hypoxia, circadian disruption promotes the development of all levels of fatty liver disease—from steatosis to cirrhosis. Vice versa, MASLD/MASH can feed back on circadian regulatory functions through the same pathways, thus generating a vicious cycle of chronometabolic dysfunction.
Clinically, sleep–wake rhythm disturbances are associated with the onset and the worsening of chronic
liver disease and negatively affect quality of life and health95. Observational studies showed that short sleep duration and poor sleep quality were associated with increased risk of MASLD in middle-aged111 and younger people112. A meta-analysis reported increased risk of MASLD in people experiencing insomnia113. Additionally, a study comparing 46 patients with histologically diagnosed MASLD to 22 healthy individuals found that MASLD patients had later sleep onset times, worse sleep quality and shorter sleep duration114. Furthermore, large cohort longitudinal studies support the relationship between sleep disturbances and duration and MASLD115,116. However, it is unknown whether sleep disturbances promote MASLD and how MASLD influences sleep. A recent bidirectional Mendelian randomisation study using genetic and sleep data found that different sleep traits could trigger and foster progression of MASLD, whereas MASLD did not appear to alter sleep traits, thereby rejecting the two-way hypothesis117.
Poor sleep quality and delayed sleep onset could affect metabolic physiology and thereby promote MASLD. Obesity, a risk factor for MASLD, is associated with fragmented daily temperature and sleep rhythms118, as well as changes in endocrine oscillations, e.g., of nocturnal melatonin119,120. Sleep disturbances can also dysregulate hormones such as ghrelin and leptin and the activity of the endocannabinoid system121,122,123. Studies in healthy individuals showed that sleep restriction increases appetite and calorie intake, decreases insulin sensitivity and alters cortisol levels124. MASLD patients are more likely than healthy patients to eat later in the day114 and go to bed later114,125. Furthermore, the combination of late chronotype with poor adherence to a Mediterranean-type diet is associated with advanced liver fibrosis in patients with MASLD126. In a study of 295,837 participants who were followed for 15 years, a U-shaped relationship between duration of sleep and the incidence of HCC and mortality from chronic liver disease was observed127. Excessive daytime sleepiness and longer daytime naps also increases MASLD risk113,128 (Table 2).
MASLD influences the physiology of peripheral organs and global physiology
Because of its central role in organising metabolism, disruption of the circadian clock is associated with obesity and insulin resistance129,130. In a clinical study, only ten days of circadian misalignment were sufficient to reduce insulin sensitivity131. Thus, insulin resistance caused by circadian disruption could contribute to the development of MASLD by stimulating adipose tissue to expand and release pro-inflammatory adipokines and free fatty acids, leading to systemic inflammation and ectopic fat accumulation132,133. This state of chronic systemic inflammation, also observed in obesity and T2D, is associated with the disruption of the circadian clock in skeletal muscles and WAT134,135, possibly because of the direct interaction between the inflammation-activated transcription factors NF-κB and BMAL1136,137. The relationship between malnutrition or sarcopenia, which is common in decompensated cirrhosis, and circadian disruption has not been well studied. In cirrhosis patients, malnutrition and sarcopenia has been attributed to the combination of abnormalities in nutrients intake, absorption and metabolism138. Albeit reasonable (please refer to ref. 7 for a review), the possibility that it may relate, at least to some extent, to liver clock dysfunction and central-peripheral clock desynchrony has remained untested.
Is the liver a hub between central and peripheral clocks?
The liver integrates rhythmic systemic cues to synchronise its physiology with nutrient cycles and food intake and transmits these signals to other tissues, including the central clock in the SCN2. Accordingly, and as previously discussed, advanced fibrosis is associated with alterations of the activity/sleep cycle and disturbed metabolism in other tissues, potentially through inter-organ communication. There is other evidence that the liver clock communicates with circadian clocks in other organs. For example, WAT and lung circadian clocks cannot entrain properly to feeding rhythms in mice with Bmal1 deleted in hepatocytes139. The liver clock is also necessary and sufficient to integrate feeding signals and restore rhythmic liver physiology in animals otherwise devoid of functional circadian clocks140. Moreover, the reconstituted liver clock in these clock-less mice also communicates with the clocks in skeletal muscles and regulates rhythmic gene expression in muscles140,141. However, the restoration of the rhythmic physiology of these tissues is only partial and functional clocks in both liver and skeletal muscles are required to completely restore glucose metabolism in condition of rhythmic feeding140,141.
The role of the liver in coordinating global physiology in animals with functional circadian systems is less clear. Mice in which mouse hepatocytes were replaced by human hepatocytes were unable to integrate signals from the mouse142. Mice with human hepatocytes had advances liver circadian clocks, similar to clock-less animals with functional circadian clocks restored only in hepatocytes143. Strikingly, this liver phase advance impacted their global physiology. Additionally, mice with human hepatocytes had altered rhythms of gene expression in the SCN and the arcuate nucleus of the hypothalamus. Their capacity to synchronise to daytime feeding was impacted. The humanised mice showed a behaviour similar to that of SCN-lesioned animals142. Considering the similarities in phenotypes, similar mechanisms could be involved in altering the phases of liver clocks in mouse models of liver fibrosis91,92 and in humans with liver cirrhosis93. Thus, it is possible that in MASH or liver cirrhosis, the liver could transmit signals to the hypothalamus to modulate diurnal activity.
The mechanism that could transmit the signal from the pathogenic liver to the brain and other organs remains elusive. One hypothesis is that abnormal circulating factors originating from the liver and specific to MASLD impact the rhythmic physiology of other central and peripheral organs. Accordingly, MASLD leads to perturbed profiles of circulating proteins and metabolites144,145. A recent study shows that disrupting the liver circadian clock by simultaneously deleting Rev-erbα and Rev-erbβ or Bmal1 specifically in hepatocytes of adult mice disrupted their feeding rhythm146. Because deletion of Rev-erbα/β or Bmal1 is associated with disrupted liver physiology139,147,148,149, this condition could recapitulate models of MASLD and mice with “humanised” livers. Surprisingly, the perturbation of the feeding rhythm was lost after vagotomy or ablation of liver vagal afferent neurons, implying a role of the autonomous nervous system (ANS). In addition, the perturbation of the feeding rhythm and body weight gain caused by HFD feeding were mitigated by liver vagotomy146. Together, these data highlight a potential critical role of the ANS in the modulation of SCN activity by the cirrhotic liver, exemplified by the fact that lipid liver accumulation modulates the activity of the ANS via the alteration of direct GABAergic connections150,151. However, this result is at odds with previous publications showing a normal feeding rhythm in hepatocyte Bmal1149,152 and Rev-erbs153 knockout mice. Moreover, this finding must be considered relative to numerous studies that showed that severing the neuronal connection between the liver and the SCN through vagotomy or liver transplantation was sufficient to induce a global loss of the diurnal feeding pattern in rodents154,155,156,157, a phenomenon not observed in this study.
Conclusion
Mounting evidence suggests there may be bidirectional connections between the liver and the central clock that regulate circadian behaviour. Circadian disruption leads to liver steatosis and MASLD with liver fibrosis is associated with alterations of circadian rhythms and sleep (Fig. 3). Accordingly, restoring a rhythmic liver physiology through dietary intervention of small molecules targeting the circadian clock appears like an interesting strategy that was successful in animal models. Reciprocally, anecdotical evidence tends to show that eliminating liver fibrosis can restore circadian rhythmicity and sleep in patients with liver cirrhosis. Recent experimental evidence suggests a role of the ANS in this inter-organ communication. Accordingly, degeneration of sympathetic liver innervation observed in MASLD could play a role in both the pathophysiology of the disease and the associated alterations of circadian behaviour110. Therefore, the liver–brain connection would deserve more attention in future research.
Disrupted circadian outputs from the diseased liver can affect metabolic and immune rhythms in other tissues. In MASH patients or animal models of fatty liver disease repercussions on rhythmic functions outside the liver have been shown for the SCN (sleep–wake cycle regulation), the arcuate nucleus (appetite regulation), metabolic tissues such as muscle (lipid and carbohydrate metabolism), and inflammatory processes in the lung and circulating immune cells.
Data Availability
No datasets were generated or analysed during the current study.
References
Hastings, M. H., Maywood, E. S. & Brancaccio, M. Generation of circadian rhythms in the suprachiasmatic nucleus. Nat. Rev. Neurosci. 19, 453–469 (2018).
Bass, J. Interorgan rhythmicity as a feature of healthful metabolism. Cell Metab. 36, 655–669 (2024).
Yamazaki, S. et al. Resetting central and peripheral circadian oscillators in transgenic rats. Science 288, 682–685 (2000).
Yoo, S. -H. et al. PERIOD2:LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc. Natl. Acad. Sci. USA 101, 5339–5346 (2004).
Jabbur, M. L., Dani, C., Spoelstra, K., Dodd, A. N. & Johnson, C. H. Evaluating the adaptive fitness of circadian clocks and their evolution. J. Biol. Rhythms 39, 115–134 https://doi.org/10.1177/07487304231219206 (2024).
Bolshette, N., Ibrahim, H., Reinke, H. & Asher, G. Circadian regulation of liver function: from molecular mechanisms to disease pathophysiology. Nat. Rev. Gastroenterol. Hepatol. 20, 695–707 (2023).
Costa, R., Mangini, C., Domenie, E. D., Zarantonello, L. & Montagnese, S. Circadian rhythms and the liver. Liver Int. 43, 534–545 (2023).
Patke, A., Young, M. W. & Axelrod, S. Molecular mechanisms and physiological importance of circadian rhythms. Nat. Rev. Mol. Cell Biol. 21, 67–84 (2020).
Atger, F. et al. Circadian and feeding rhythms differentially affect rhythmic mRNA transcription and translation in mouse liver. Proc. Natl. Acad. Sci. USA 112, E6579–E6588 (2015).
Greenwell, B. J. et al. Rhythmic food intake drives rhythmic gene expression more potently than the hepatic circadian clock in mice. Cell Rep. 27, 649–657.e645 (2019).
Mange, F. et al. Diurnal regulation of RNA polymerase III transcription is under the control of both the feeding–fasting response and the circadian clock. Genome Res. 27, 973–984 (2017).
Sinturel, F. et al. Diurnal oscillations in liver mass and cell size accompany ribosome assembly cycles. Cell 169, 651–663.e614 (2017).
Vollmers, C. et al. Time of feeding and the intrinsic circadian clock drive rhythms in hepatic gene expression. Proc. Natl. Acad. Sci. USA 106, 21453–21458 (2009).
Weger, B. D. et al. Systematic analysis of differential rhythmic liver gene expression mediated by the circadian clock and feeding rhythms. Proc. Natl. Acad. Sci. USA 118, e2015803118 (2021).
Damiola, F. et al. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 14, 2950–2961 (2000).
Stokkan, K. -A., Yamazaki, S., Tei, H., Sakaki, Y. & Menaker, M. Entrainment of the circadian clock in the liver by feeding. Science 291, 490–493 (2001).
Roenneberg, T. & Merrow, M. The circadian clock and human health. Curr. Biol. 26, R432–R443 (2015).
Ando, H., Ushijima, K. & Fujimura, A. Indirect effects of glucagon-like peptide-1 receptor agonist exendin-4 on the peripheral circadian clocks in mice. PLoS One 8, e81119 (2013).
Crosby, P. et al. Insulin/IGF-1 drives PERIOD synthesis to entrain circadian rhythms with feeding time. Cell 177, 896–909.e820 (2019).
Landgraf, D. et al. Oxyntomodulin regulates resetting of the liver circadian clock by food. Elife 4, e06253 (2015).
Tahara, Y., Otsuka, M., Fuse, Y., Hirao, A. & Shibata, S. Refeeding after fasting elicits insulin-dependent regulation of Per2 and Rev-erbα with shifts in the liver clock. J. Biol. Rhythms 26, 230–240 (2011).
Ikeda, Y. et al. Glucagon and/or IGF-1 production regulates resetting of the liver circadian clock in response to a protein or amino acid-only diet. EBiomedicine 28, 210–224 (2018).
Mukherji, A., Kobiita, A. & Chambon, P. Shifting the feeding of mice to the rest phase creates metabolic alterations, which, on their own, shift the peripheral circadian clocks by 12 h. Proc. Natl. Acad. Sci. USA 112, E6683–E6690 (2015).
Ando, H., et al. Sustained effect of habitual feeding time on daily rhythm of core body temperature in mice. Front Nutr. 9, 966788 (2022).
Shimatani, H. et al. Thermographic imaging of mouse across circadian time reveals body surface temperature elevation associated with non-locomotor body movements. PLoS One 16, e0252447 (2021).
Brown, S. A., Zumbrunn, G., Fleury-Olela, F., Preitner, N. & Schibler, U. Rhythms of mammalian body temperature can sustain peripheral circadian clocks. Curr. Biol. 12, 1574–1583 (2002).
Buhr, E. D., Yoo, S. -H. & Takahashi, J. S. Temperature as a universal resetting cue for mammalian circadian oscillators. Science 330, 379–385 (2010).
Fischl, H., et al. Cold-induced chromatin compaction and nuclear retention of clock mRNAs resets the circadian rhythm. EMBO J. 39, e105604 (2020).
Morf, J. et al. Cold-inducible RNA-binding protein modulates circadian gene expression posttranscriptionally. Science 338, 379–383 (2012).
Karlsen, T. H. et al. The EASL–lancet liver commission: protecting the next generation of Europeans against liver disease complications and premature mortality. Lancet 399, 61–116 (2022).
Rinella, M. E. et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology 78, 1966–1986 (2023).
Satapathy, S. K. & Sanyal, A. J. Epidemiology and natural history of nonalcoholic fatty liver disease. Semin Liver Dis. 35, 221–235 (2015).
Chew, N. W. S. et al. The global burden of metabolic disease: data from 2000 to 2019. Cell Metab. 35, 414–428.e413 (2023).
Younossi, Z. M., Kalligeros, M. & Henry, L. Epidemiology of metabolic dysfunction-associated steatotic liver disease. Clin. Mol. Hepatol. https://doi.org/10.3350/cmh.2024.0431 (2024).
Croci, I. et al. Non-alcoholic fatty liver disease: prevalence and all-cause mortality according to sedentary behaviour and cardiorespiratory fitness. The HUNT study. Prog. Cardiovasc Dis. 62, 127–134 (2019).
Jou, J., Choi, S. S. & Diehl, A. M. Mechanisms of disease progression in nonalcoholic fatty liver disease. Semin Liver Dis. 28, 370–379 (2008).
Abdelmalek, M. F. et al. Increased fructose consumption is associated with fibrosis severity in patients with nonalcoholic fatty liver disease. Hepatology 51, 1961–1971 (2010).
Geidl-Flueck, B. et al. Fructose- and sucrose- but not glucose-sweetened beverages promote hepatic de novo lipogenesis: a randomized controlled trial. J. Hepatol. 75, 46–54 (2021).
Fonken, L. K. et al. Light at night increases body mass by shifting the time of food intake. Proc. Natl. Acad. Sci. USA 107, 18664–18669 (2010).
Yue, F. et al. Effects of constant light exposure on sphingolipidomics and progression of NASH in high-fat-fed rats. J. Gastroenterol. Hepatol. 35, 1978–1989 (2020).
Salgado-Delgado, R. C. et al. Shift work or food intake during the rest phase promotes metabolic disruption and desynchrony of liver genes in male rats. PLoS One 8, e60052 (2013).
Anderson, S. T. et al. Sexual dimorphism in the response to chronic circadian misalignment on a high-fat diet. Sci. Transl. Med 15, eabo2022 (2023).
Kettner, N. M. et al. Circadian homeostasis of liver metabolism suppresses hepatocarcinogenesis. Cancer Cell 30, 909–924 (2016).
Padilla, J. et al. Circadian dysfunction induces NAFLD-related human liver cancer in a mouse model. J. Hepatol. 80, 282–292 (2024).
Jacobi, D. et al. Hepatic Bmal1 regulates rhythmic mitochondrial dynamics and promotes metabolic fitness. Cell Metab. 22, 709–720 (2015).
Zhang, D. et al. The hepatic BMAL1/AKT/lipogenesis axis protects against alcoholic liver disease in mice via promoting PPARα pathway. Hepatology 68, 883–896 (2018).
Yang, T. et al. NPAS2 contributes to liver fibrosis by direct transcriptional activation of Hes1 in hepatic stellate cells. Mol. Ther. Nucleic Acids 18, 1009–1022 (2019).
Jouffe, C. et al. Disruption of the circadian clock component BMAL1 elicits an endocrine adaption impacting on insulin sensitivity and liver disease. Proc. Natl. Acad. Sci. USA 119, e2200083119 (2022).
Zhan, C. et al. BMAL1 deletion protects against obesity and non-alcoholic fatty liver disease induced by a high-fat diet. Int J. Obes.48, 469–476 (2023).
Yu, F. et al. Deficiency of intestinal Bmal1 prevents obesity induced by high-fat feeding. Nat. Commun. 12, 5323 (2021).
Anafi, R. C., Francey, L. J., Hogenesch, J. B. & Kim, J. CYCLOPS reveals human transcriptional rhythms in health and disease. Proc. Natl. Acad. Sci. USA 114, 5312–5317 (2017).
Johanns, M. et al. Time-of-day-dependent variation of the human liver transcriptome and metabolome is disrupted in MASLD. JHEP Rep. 6, 100948 (2024).
Balakrishnan, M., El-Serag, H. B., Kanwal, F. & Thrift, A. P. Shiftwork is not associated with increased risk of NAFLD: findings from the national health and nutrition examination survey. Dig. Dis. Sci. 62, 526–533 (2017).
Zhang, S. et al. Rotating night shift work and non-alcoholic fatty liver disease among steelworkers in China: a cross-sectional survey. Occup. Environ. Med 77, 333–339 (2020).
Wang, F. et al. Night shift work and abnormal liver function: is non-alcohol fatty liver a necessary mediator? Occup. Environ. Med 76, 83–89 (2019).
Huang, H., Liu, Z., Xie, J. & Xu, C. Association between night shift work and NAFLD: a prospective analysis of 281,280 UK biobank participants. BMC Public Health 23, 1282 (2023).
Maidstone, R., Rutter, M. K., Marjot, T., Ray, D. W. & Baxter, M. Shift work and evening chronotype are associated with hepatic fat fraction and non-alcoholic fatty liver disease in 282,303 UK biobank participants. Endocr. Connect 13, e230472 (2024).
Gu, F. et al. Longitude position in a time zone and cancer risk in the United States. Cancer Epidemiol. Biomark. Prev. 26, 1306–1311 (2017).
VoPham, T. et al. Circadian misalignment and hepatocellular carcinoma incidence in the United States. Cancer Epidemiol. Biomark. Prev. 27, 719–727 (2018).
Veasey, S. C. & Rosen, I. M. Obstructive sleep apnea in adults. N. Engl. J. Med. 380, 1442–1449 (2019).
Martinez-Nicolas, A., et al. Ambulatory circadian monitoring in sleep disordered breathing patients and CPAP treatment. Sci. Rep. 11, 14711 (2021).
Fu, Y. et al. Chronic intermittent hypoxia contributes to non-alcoholic steatohepatitis progression in patients with obesity. Hepatol. Int. 16, 824–834 (2022).
Jullian-Desayes, I. et al. Obstructive sleep apnea, chronic obstructive pulmonary disease and NAFLD: an individual participant data meta-analysis. Sleep. Med. 77, 357–364 (2021).
Mesarwi, O. A., Loomba, R. & Malhotra, A. Obstructive sleep apnea, hypoxia, and nonalcoholic fatty liver disease. Am. J. Respir. Crit. Care Med. 199, 830–841 (2019).
Parikh, M. P., Gupta, N. M. & McCullough, A. J. Obstructive sleep apnea and the liver. Clin. Liver Dis. 23, 363–382 (2019).
Koritala, B. S. C. et al. Obstructive sleep apnea in a mouse model is associated with tissue-specific transcriptomic changes in circadian rhythmicity and mean 24-hour gene expression. PLoS Biol. 21, e3002139 (2023).
Manella, G. et al. Hypoxia induces a time- and tissue-specific response that elicits intertissue circadian clock misalignment. Proc. Natl. Acad. Sci. USA 117, 779–786 (2020).
Chaix, A., Zarrinpar, A., Miu, P. & Panda, S. Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab. 20, 991–1005 (2014).
Hatori, M. et al. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 15, 848–860 (2012).
He, B. et al. The small molecule nobiletin targets the molecular oscillator to enhance circadian rhythms and protect against metabolic syndrome. Cell Metab. 23, 610–621 (2016).
Larion, S. et al. The biological clock enhancer nobiletin ameliorates steatosis in genetically obese mice by restoring aberrant hepatic circadian rhythm. Am. J. Physiol. Gastrointest. Liver Physiol. 323, G387–G400 (2022).
Ni, Y. et al. Pharmacological activation of REV-ERBα improves nonalcoholic steatohepatitis by regulating intestinal permeability. Metabolism 114, 154409 (2021).
Wei, X. et al. Effects of time-restricted eating on nonalcoholic fatty liver disease: the TREATY-FLD randomized clinical trial. JAMA Netw. Open 6, e233513 (2023).
Maruthur, N. M. et al. Effect of isocaloric, time-restricted eating on body weight in adults with obesity. Ann. Intern Med 177, 549–558 (2024).
Quist, J. S. et al. Effects of 3 months of 10-h per-day time-restricted eating and 3 months of follow-up on bodyweight and cardiometabolic health in Danish individuals at high risk of type 2 diabetes: the RESET single-centre, parallel, superiority, open-label, randomised controlled trial. Lancet Healthy Longev. 5, e314–e325 (2024).
Liu, D. et al. Calorie restriction with or without time-restricted eating in weight loss. N. Engl. J. Med. 386, 1495–1504 (2022).
Ito, M. et al. Longitudinal analysis of murine steatohepatitis model induced by chronic exposure to high-fat diet. Hepatol. Res. 37, 50–57 (2007).
Dyar, K. A. et al. Atlas of circadian metabolism reveals system-wide coordination and communication between clocks. Cell 174, 1571–1585.e1511 (2018).
Kohsaka, A. et al. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab. 6, 414–421 (2007).
Deota, S. et al. Diurnal transcriptome landscape of a multi-tissue response to time-restricted feeding in mammals. Cell Metab. 35, 150–165.e154 (2023).
Pendergast, J. S. et al. High-fat diet acutely affects circadian organisation and eating behavior. Eur. J. Neurosci. 37, 1350–1356 (2013).
Turek, F. W. et al. Obesity and metabolic syndrome in circadian clock mutant mice. Science 308, 1043–1045 (2005).
Ho, A. & Chin, A. Circadian feeding and drinking patterns of genetically obese mice fed solid chow diet. Physiol. Behav. 43, 651–656 (1988).
Ando, H. et al. Impairment of peripheral circadian clocks precedes metabolic abnormalities in ob/ob mice. Endocrinology 152, 1347–1354 (2011).
Kudo, T. et al. Night-time restricted feeding normalises clock genes and Pai-1 gene expression in the db/db mouse liver. Diabetologia 47, 1425–1436 (2004).
Branecky, K. L., Niswender, K. D. & Pendergast, J. S. Disruption of daily rhythms by high-fat diet is reversible. PLoS ONE 10, e0137970 (2015).
Buckley, T. N. et al. High-fat feeding disrupts daily eating behavior rhythms in obesity-prone but not in obesity-resistant male inbred mouse strains. Am. J. Physiol. Regul. Integr. Comp. Physiol. 320, R619–R629 (2021).
Omotola, O., Legan, S., Slade, E., Adekunle, A. & Pendergast, J. S. Estradiol regulates daily rhythms underlying diet-induced obesity in female mice. Am. J. Physiol. Endocrinol. Metab. 317, E1172–E1181 (2019).
Mendoza, J., Pévet, P. & Challet, E. High-fat feeding alters the clock synchronization to light. J. Physiol. 586, 5901–5910 (2008).
Matsumoto, M. et al. An improved mouse model that rapidly develops fibrosis in non-alcoholic steatohepatitis. Int J. Exp. Pathol. 94, 93–103 (2013).
de Assis, L. V. M., Demir, M. & Oster, H. Nonalcoholic steatohepatitis disrupts diurnal liver transcriptome rhythms in mice. Cell Mol. Gastroenterol. Hepatol. 16, 341–354 (2023).
Leclère, P. S. et al. MCD diet-induced steatohepatitis generates a diurnal rhythm of associated biomarkers and worsens liver injury in Klf10 deficient mice. Sci. Rep. 10, 12139 (2020).
Montagnese, S. et al. Sleep-wake abnormalities in patients with cirrhosis. Hepatology 59, 705–712 (2014).
Córdoba, J. et al. High prevalence of sleep disturbance in cirrhosis. Hepatology 27, 339–345 (1998).
Marjot, T., Ray, D. W., Williams, F. R., Tomlinson, J. W. & Armstrong, M. J. Sleep and liver disease: a bidirectional relationship. Lancet Gastroenterol. Hepatol. 6, 850–863 (2021).
Montagnese, S., Middleton, B., Mani, A. R., Skene, D. J. & Morgan, M. Y. Sleep and circadian abnormalities in patients with cirrhosis: features of delayed sleep phase syndrome? Metab. Brain Dis. 24, 427–439 (2009).
Montagnese, S., Middleton, B., Skene, D. J. & Morgan, M. Y. Night-time sleep disturbance does not correlate with neuropsychiatric impairment in patients with cirrhosis. Liver Int. 29, 1372–1382 (2009).
Bersagliere, A. et al. Induced hyperammonemia may compromise the ability to generate restful sleep in patients with cirrhosis. Hepatology 55, 869–878 (2012).
Sherlock, S., Summerskill, W. H. J., White, L. P. & Phear, E. A. Portal-systemic encephalopathy neurological complications of liver disease. Lancet 264, 453–457 (1954).
Laidlaw, J., Read, A. E. & Sherlock, S. Morphine tolerance in hepatic cirrhosis. Gastroenterology 40, 389–396 (1961).
Boy, C. et al. Cerebral A1 adenosine receptors (A1AR) in liver cirrhosis. Eur. J. Nucl. Med. Mol. Imaging 35, 589–597 (2008).
Marini, S. et al. Abnormalities in the polysomnographic, adenosine and metabolic response to sleep deprivation in an animal model of hyperammonemia. Front. Physiol. 8, 636 (2017).
Montagnese, S., Middleton, B., Mani, A. R., Skene, D. J. & Morgan, M. Y. On the origin and the consequences of circadian abnormalities in patients with cirrhosis. Am. J. Gastroenterol. 105, 1773–1781 (2010).
Steindl, P. E. et al. Disruption of the diurnal rhythm of plasma melatonin in cirrhosis. Ann. Intern. Med. 123, 274–277 (1995).
Montagnese, S., Middleton, B., Mani, A. R., Skene, D. J. & Morgan, M. Y. Changes in the 24-h plasma cortisol rhythm in patients with cirrhosis. J. Hepatol. 54, 588–590 (2011).
Steindl, P. E., Ferenci, P. & Marktl, W. Impaired hepatic catabolism of melatonin in cirrhosis. Ann. Intern. Med. 127, 494 (1997).
Cordoba, J., Steindl, P. & Blei, A. T. Melatonin arrhythmia is corrected after liver transplantation. Am. J. Gastroenterol. 104, 1862–1863 (2009).
Hutchison, A. L., Tavaglione, F., Romeo, S. & Charlton, M. Endocrine aspects of metabolic dysfunction-associated steatotic liver disease (MASLD): Beyond insulin resistance. J. Hepatol. 79, 1524–1541 (2023).
Horn, P. & Tacke, F. Metabolic reprogramming in liver fibrosis. Cell Metab. 36, 1439–1455 (2024).
Adori, C. et al. Disorganization and degeneration of liver sympathetic innervations in nonalcoholic fatty liver disease revealed by 3D imaging. Sci. Adv. 7, eabg5733 (2021).
Kim, C. -W. et al. Sleep duration and quality in relation to non-alcoholic fatty liver disease in middle-aged workers and their spouses. J. Hepatol. 59, 351–357 (2013).
Trovato, F. M. et al. Fatty liver disease and lifestyle in youngsters: diet, food intake frequency, exercise, sleep shortage and fashion. Liver Int. 36, 427–433 (2016).
Wijarnpreecha, K., Thongprayoon, C., Panjawatanan, P. & Ungprasert, P. Insomnia and risk of nonalcoholic fatty liver disease: a systematic review and meta-analysis. J. Postgrad. Med. 63, 226–231 (2017).
Bernsmeier, C. et al. Sleep disruption and daytime sleepiness correlating with disease severity and insulin resistance in non-alcoholic fatty liver disease: a comparison with healthy controls. PLoS ONE 10, e0143293 (2015).
Okamura, T. et al. Short sleep duration is a risk of incident nonalcoholic fatty liver disease: a population-based longitudinal study. J. Gastrointestin Liver Dis. 28, 73–81 (2019).
Um, Y. J. et al. Sleep duration, sleep quality, and the development of nonalcoholic fatty liver disease: a cohort study. Clin. Transl. Gastroenterol. 12, e00417 (2021).
Sun, Z. et al. Causal relationship between nonalcoholic fatty liver disease and different sleep traits: a bidirectional Mendelian randomized study. Front Endocrinol. 14, 1159258 (2023).
Corbalán-Tutau, M. D. et al. Differences in daily rhythms of wrist temperature between obese and normal-weight women: associations with metabolic syndrome features. Chronobiol. Int. 28, 425–433 (2011).
Corbalán-Tutau, D., Madrid, J. A., Nicolás, F. & Garaulet, M. Daily profile in two circadian markers “melatonin and cortisol” and associations with metabolic syndrome components. Physiol. Behav. 123, 231–235 (2014).
Mäntele, S. et al. Daily rhythms of plasma melatonin, but not plasma leptin or leptin mrna, vary between lean, obese and type 2 diabetic men. PLoS ONE 7, e37123 (2012).
Spiegel, K. et al. Leptin levels are dependent on sleep duration: relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. J. Clin. Endocrinol. Metab. 89, 5762–5771 (2004).
Hanlon, E. C. et al. Sleep restriction enhances the daily rhythm of circulating levels of endocannabinoid 2-arachidonoylglycerol. Sleep 39, 653–664 (2016).
Taheri, S., Lin, L., Austin, D., Young, T. & Mignot, E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 1, e62 (2004).
Perez-Diaz-del-Campo, N. et al. Role of circadian clock on the pathogenesis and lifestyle management in non-alcoholic fatty liver disease. Nutrients 14, 5053 (2022).
Zhou, J., Long, Y., Ding, N. & Su, Y. Association between bedtime at night and nonalcoholic fatty liver disease diagnosed by liver ultrasound transient elastography. Diab. Res. Clin. Pract. 184, 109195 (2022).
Castelnuovo, G. et al. Impact of chronotype and mediterranean diet on the risk of liver fibrosis in patients with non-alcoholic fatty liver disease. Nutrients 15, 3257 (2023).
Long, L., et al. Daytime napping, nighttime sleeping duration, and risk of hepatocellular carcinoma and liver disease-related mortality. JHEP Rep. 5, 100819 (2023).
Peng, K. et al. Short sleep duration and longer daytime napping are associated with non-alcoholic fatty liver disease in Chinese adults. J. Diab. 9, 827–836 (2017).
Chaput, J. -P. et al. The role of insufficient sleep and circadian misalignment in obesity. Nat. Rev. Endocrinol. 19, 82–97 (2023).
Stenvers, D. J., Scheer, F. A. J. L., Schrauwen, P., la Fleur, S. E. & Kalsbeek, A. Circadian clocks and insulin resistance. Nat. Rev. Endocrinol. 15, 75–89 (2019).
Scheer, F. A. J. L., Hilton, M. F., Mantzoros, C. S. & Shea, S. A. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc. Natl. Acad. Sci. USA 106, 4453–4458 (2009).
Saponaro, C. et al. Adipose tissue dysfunction and visceral fat are associated with hepatic insulin resistance and severity of NASH even in lean individuals. Liver Int. 42, 2418–2427 (2022).
Sears, B. & Perry, M. The role of fatty acids in insulin resistance. Lipids Health Dis. 14, 121 (2015).
Gabriel, B. M. et al. Disrupted circadian oscillations in type 2 diabetes are linked to altered rhythmic mitochondrial metabolism in skeletal muscle. Sci. Adv. 7, eabi9654 (2021).
Maury, E., Navez, B. & Brichard, S. M. Circadian clock dysfunction in human omental fat links obesity to metabolic inflammation. Nat. Commun. 12, 2388 (2021).
Hong, H. -K. et al. Requirement for NF-κB in maintenance of molecular and behavioral circadian rhythms in mice. Genes Dev. 32, 1367–1379 (2018).
Shen, Y. et al. NF-κB modifies the mammalian circadian clock through interaction with the core clock protein BMAL1. PLoS Genet 17, e1009933 (2021).
Tandon, P., Raman, M., Mourtzakis, M. & Merli, M. A practical approach to nutritional screening and assessment in cirrhosis. Hepatology 65, 1044–1057 (2017).
Manella, G. et al. The liver-clock coordinates rhythmicity of peripheral tissues in response to feeding. Nat. Metab. https://doi.org/10.1038/s42255-021-00395-7 (2021).
Greco, C. M. et al. Integration of feeding behavior by the liver circadian clock reveals network dependency of metabolic rhythms. Sci. Adv. 7, eabi7828 (2021).
Smith, J. G. et al. Liver and muscle circadian clocks cooperate to support glucose tolerance in mice. Cell Rep. 42, 112588 (2023).
Delbès, A. -S. et al. Mice with humanized livers reveal the role of hepatocyte clocks in rhythmic behavior. Sci. Adv. 9, eadf2982 (2023).
Koronowski, K. B. et al. Defining the independence of the liver circadian clock. Cell 177, 1448–1462 (2019).
Niu, L. et al. Plasma proteome profiling discovers novel proteins associated with non-alcoholic fatty liver disease. Mol. Syst. Biol. 15, e8793 (2019).
Masoodi, M. et al. Metabolomics and lipidomics in NAFLD: biomarkers and non-invasive diagnostic tests. Nat. Rev. Gastroenterol. Hepatol. 18, 835–856 (2021).
Woodie, L. N. et al. Hepatic vagal afferents convey clock-dependent signals to regulate circadian food intake. Science 386, 673–677 (2024).
Bugge, A. et al. Rev-erbα and Rev-erbβ coordinately protect the circadian clock and normal metabolic function. Genes Dev. 26, 657–667 (2012).
Cho, H. et al. Regulation of circadian behaviour and metabolism by REV-ERB-a and REV-ERB-b. Nature 485, 123–127 (2012).
Lamia, K. A., Storch, K. -F. & Weitz, C. J. Physiological significance of a peripheral tissue circadian clock. Proc. Natl. Acad. Sci. USA 105, 15172–15177 (2008).
Geisler, C. E. et al. Hepatocyte membrane potential regulates serum insulin and insulin sensitivity by altering hepatic GABA release. Cell Rep. 35, 109298. https://doi.org/10.1016/j.celrep.2021.109298 (2021).
Kalsbeek, A., La Fleur, S., Van Heijningen, C. & Buijs, R. M. Suprachiasmatic GABAergic inputs to the paraventricular nucleus control plasma glucose concentrations in the rat via sympathetic innervation of the liver. J. Neurosci. 24, 7604–7613 (2004).
Johnson, B. P. et al. Hepatocyte circadian clock controls acetaminophen bioactivation through NADPH-cytochrome P450 oxidoreductase. Proc. Natl. Acad. Sci. USA 111, 18757–18762 (2014).
Guan, D. et al. The hepatocyte clock and feeding control chronophysiology of multiple liver cell types. Science 369, 1388–1394 (2020).
Friedman, M. I. & Sawchenko, P. E. Evidence for hepatic involvement in control of ad libitum food intake in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 247, R106–R113 (1984).
Louis-Sylvestre, J., Larue-Achagiotis, C., Michel, A. & Houssin, D. Feeding pattern of liver-transplanted rats. Physiol. Behav. 48, 321–326 (1990).
Louis-Sylvestre, J. Feeding and metabolic patterns in rats with truncular vagotomy or with transplanted beta-cells. Am. J. Physiol. 235, E119–E125 (1978).
Itoh, S., Katsuura, G. & Hirota, R. Diminished circadian rhythm of locomotor activity after vagotomy in rats. Jpn J. Physiol. 31, 957–961 (1981).
Acknowledgements
This work was supported by grants from the National Health and Medical Research Council (Australia) Synergy Grant (2019260 to F.G.); National Institute of Health (NIH) (R01AG078241 to F.G., R01DK124774 to J.S.P.); the Novo Nordisk Foundation (Hallas-Møller Ascending Investigator grant #0087882 to F.G.); the National Science Foundation (NSF) (IOS-2045267 to J.S.P); the Relevant Researches of National Interest (PRIN) 2022 Next Generation EU (2022L273C9 to E.B. and 2022N2KJAM (CUP E53D23013280006) to S.M.); the Deutsche Forschungsgemeinschaft (DFG) Collaborative Research Centres (CRC 296 LocoTact-TP13 to H.O.); and the European Union Horizon 2020 H2020-SC1-BHC-2018-2020 (847949 to S.M.). The figures were created with BioRender.com.
Author information
Authors and Affiliations
Contributions
F.G., E.B., G.C., H.O., J.S.P., and S.M.: Conceptualisation; Writing—original draft; F.G., E.B., G.C., H.O., J.S.P., and S.M.: Writing—Review & editing; F.G., E.B., G.C., H.O., J.S.P., and S.M.: visualisation. All authors have read and approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Gachon, F., Bugianesi, E., Castelnuovo, G. et al. Potential bidirectional communication between the liver and the central circadian clock in MASLD. npj Metab Health Dis 3, 15 (2025). https://doi.org/10.1038/s44324-025-00058-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s44324-025-00058-1