Abstract
Coronary biomechanics including structural wall stress and strain on the vessel wall and flow wall shear stress on the endothelial surface play a vital role in plaque progression, rupture and erosion. This review summarizes recent advances in coronary intravascular imaging, image-based biomechanical modeling, and their applications in investigating possible biomechanical mechanisms of plaque rupture and erosion, and developing interventional therapies targeting unfavorable mechanical conditions for personalized treatment and precision medicine.
Similar content being viewed by others
Introduction
Acute coronary syndrome (ACS) is a major cause of morbidity and mortality worldwide, responsible for an estimated 20% of all deaths from cardiovascular diseases (CVD) according to a survey of 122 countries in 20201,2. ACS is characterized by a sudden decrease or block of blood supply to the heart, usually presenting with abrupt onset of a group of critical cardiovascular conditions including non-ST-elevation myocardial infarction (NSTEMI), ST-elevation myocardial infarction (STEMI) and unstable angina3,4,5. The pathophysiological mechanisms of ACS are multifaceted6, including non-atherosclerotic related causes7,8 and atherosclerotic related causes9. Among them, vulnerable plaque rupture and erosion followed by the secondary thrombosis constitute about 55-60% and 30-35% of ACS cases, respectively10,11,12. Understanding these mechanisms to trigger these deleterious plaque behaviors causing ACS is of vital importance in early prevention of ACS, development of novel therapies, and clinical decision-making for interventional cardiologists13,14.
Clinical and autopsy studies have shown that there are dramatic differences in morphological characteristics between ruptured and eroded plaques15. Postmortem studies have revealed that plaque rupture is characterized by disrupted thin fibrous cap with luminal thrombus communicating with the underlying necrotic core (Fig. 1(a))16,17. The advanced plaque with the morphological characteristics of a large eccentric lipid core and thin fibrous cap infiltrated by macrophages was believed to be the precursor lesion of ruptured plaque, and termed as thin-cap fibroatheromas (TCFA)18,19. Compared to plaque rupture, plaque erosion, characterized by loss of the endothelium with overlying platelet-rich thrombus, contains an intact fibrous cap20,21. The fibrous cap is a proteoglycans- and hyaluronan-rich matrix with more smooth muscle cells, but less inflammation infiltration (Fig. 1(b))22,23. Furthermore, plaque rupture preferentially occurs at the proximal or the most stenotic region of the atherosclerotic lesion in left anterior descending (LAD) artery and right coronary artery, but rarely at the distal region24,25,26. However, most of eroded plaques locate at the distal to the atherosclerotic stenosis and near arterial bifurcation, where disturbed flow prevails26. In three major coronary arteries, erosion primarily occurs in the LAD artery, but least likely in left circumflex artery26,27,28. The compositions of the intraluminal thrombus in two cases are also different. In vivo intravascular imaging data indicate that thrombus in erosion tends to be platelet-rich white clot while that in rupture is more fibrin- and erythrocyte-rich27,29. These findings suggest that plaque rupture and erosion are two distinct pathophysiological processes with different etiologies14,22.
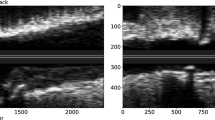
Illustration of cross-sections of ruptured plaque (a) and eroded plaque (b) with intraluminal thrombosis. In vivo optical coherence tomography images of atherosclerotic plaque rupture with a large emptied cavity (asterisks) communicating with the vessel lumen (c), and plaque erosion with a white thrombus rich in platelets (arrowheads) attached at the site of erosion (d).
The natural evolution of atherosclerotic plaque including its initiation, progression and eventually rupture and erosion is a complex process, and is greatly influenced by the mechanical forces in the coronary artery13,16. Even though progress has been made regarding to morphological and biological characteristics of these plaques, the precise mechanical mechanisms triggering plaque rupture and erosion remain somewhat elusive30,31. With considerable advance in the medical imaging technology and computational modeling techniques, it is possible for us to investigate the impacts of mechanical forces on these deleterious plaque behaviors in vivo32. In this review, we will summarize the current biomechanical studies on plaque rupture and erosion based on intravascular images, and describe our current understanding of roles of mechanical forces involved in the genesis of these plaque behaviors from computational biomechanical perspectives, to guide the better clinical management of these two mechanisms of ACS for personalized treatment and precision medicine14,33.
Biomechanical forces in coronary artery
Coronary arteries are constantly subjected to the cyclic biomechanical forces generated by the interactions between pulsatile blood flow and arterial vessel wall motion13,34. It is necessary to briefly review the concepts of these biomechanical forces, prior to the understanding of their effects on the coronary plaque. Stress is defined as the force acting on a surface per unit area35. There are three main mechanical stresses, that is, the mechanical stresses in circumferential, radial, and axial directions to deform vascular cells, atherosclerotic tissue, and the artery wall (Fig. 2). Circumferential stress is the most commonly investigated stress in the plaque research, mainly arising from the hydrostatic pressure to render the arterial expansion or contraction36,37. As a rough estimation, Laplace’s law was used to calculate the circumferential stress under the assumption that coronary artery is a straight thin-wall tube38. In normal arteries, circumferential stress is evenly distributed along the radial direction of the vessel wall39,40, with average value varying from 100 to 200 kPa33, which may increase to around 300 kPa causing plaque rupture in the atherosclerotic artery36. Similar to stress, strain is defined as the amount of deformation by a structure as a consequence of an external applied force38. In the one-dimensional situation, strain reflects changes in the length of the material over the initial length. In atherosclerotic arteries, the plaque exhibits higher strain distribution, which is the consistent with the site of plaque rupture38.
a Pulsatile blood pressure and flow velocity to induce wall shear stress; b Circumferential stress arises from radial expansion and recoil over the cardiac cycle, and Laplace’s law to estimate the wall stress, where r is vessel radius, h is the wall thickness; c The wall stress in axial, circumferential and radial directions.
Wall shear stress (WSS) is a measure of tangential frictional force per unit area acting on the endothelial surface by blood flow41. In a simple case of laminar flow in a rigid cylinder tube, the magnitude of WSS is directly proportional to mean flow velocity and plasma viscosity, but inversely proportional to vessel diameter according to Poiseuille flow42. In the realistic situation, the magnitude of WSS relies on the arterial wall motion, blood flow velocity, and plasma viscosity34, and is very sensitive to the local coronary artery geometry41. Average WSS value varies from 1 to 7 Pa (corresponding to 10 to 70 dynes/cm2) in the arterial vascular system under the physiological conditions43. However, the hemodynamic conditions were disturbed at some anatomical sites, such as outer wall of bifurcations and inner curved wall of arterial segments44, inducing low WSS. Early atherosclerotic plaques preferentially form in these regions as a result of flow-induced endothelial injury and arterial inflammation43. Other hemodynamic factors, such as time-averaged wall shear stress, and oscillatory shear index among others, are also investigated to influence the plaque initiation and development, but they are less explored in the plaque rupture and erosion studies. Comprehensive reviews of these hemodynamic factors could be found in previous excellent reviews41,45.
Decades of investigations have demonstrated the critical role of biomechanical forces during the arterial physiology or atherosclerotic pathology13. Unfortunately, these biomechanical stresses have been difficult to measure experimentally under in vivo state, putting a barrier to thoroughly understand the function of the wall stress and shear stress46,47. With the rapid advances in intravascular imaging, image-based computational models of the coronary arteries could incorporate all the relevant information to recapitulate these mechanical forces in a patient-specific setting, and examine their association with the plaque behaviors, particularly plaque rupture and erosion30,38.
Intravascular imaging
Historically, the morphological descriptions of atherosclerotic lesions underlying acute coronary syndromes were originally provided by autopsy studies16,48. They provided detailed morphological information to distinguish different types of plaque substrates related to ACS including plaque rupture49, plaque erosion26, and calcified nodule50, which have established the foundation for the in vivo diagnosis of the plaques in clinical setting51. For imaging plaque morphology in vivo, intravascular ultrasound (IVUS) and optical coherence tomography (OCT) exhibit superior performances over other noninvasive imaging technologies in identifying these plaque types, as they could reliably detect the vessel boundaries and various plaque compositions52,53. IVUS can visualize deep vessel wall structure with a penetration depth of 4-8 mm, whereas OCT can only provide superficial structures because the penetration depth of light into the vessel wall is about 1-2 mm54. However, with its 100–200 μm resolution, IVUS cannot reliably detect vulnerable plaques with thin cap thickness (threshold value 65 μm). OCT, given its superior imaging resolution (10-20 μm), possesses higher ability in visualizing the luminal surface of the vessel and the microstructure of atherosclerotic plaque such as fibrous cap, thrombus, and calcifications55,56,57. These advantages make it the most suitable imaging modality to assess plaque erosion and fibrous cap disruption in plaque rupture58,59. In a retrospective study based on 112 patients with STEMI who had undergone both OCT and IVUS, IVUS could only detect 17 plaque rupture sites among the 72 OCT-detected plaque rupture60.
Recently, a commonly accepted classification scheme has been proposed by Jia et al.51 to classify three pathological substrates causing ACS based on in vivo OCT images. In this scheme, plaque rupture was defined as a discontinuity in the fibrous cap with a communication between lumen and a plaque cavity formed due to the loss of lipid-rich necrotic core (Fig. 1(c))61. Plaque erosion could be determined if a visualized plaque with intact fibrous cap underlying a luminal thrombus is present (Fig. 1(d))62. A plaque with irregular luminal surface without the presence of any thrombus, or attenuation of underlying plaque by thrombus and no superficial lipid or calcification immediately adjacent to the thrombus site were also defined as probable erosion51. This could serve as a basis to reconstruct image-based computational models to simulate the coronary biomechanics in vivo to investigate their impact on plaque behaviors11,63,64.
Image-based biomechanical modeling
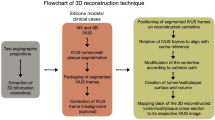
The theoretical basis of the computational simulation is that coronary biomechanical stresses from the solid and fluid motion could be accurately described by the mathematical equations, and solved by finite element approach33,34. In vivo medical image-based computational models have been employed to simulate the biomechanical stresses in both coronary plaques-wall and blood flow, to investigate the mechanical mechanisms of coronary atherosclerosis46,47. The computational models could be categorized into three types with their advantages and limitations provided in the previous references46,54: 1) Computational fluid dynamics (CFD) model only considers the motion of the blood flow, and uses Navier-Stokes equations as the governing equations to simulate wall shear stress, fluid velocity, pressure, and other hemodynamic factors41. 2) Computational solid mechanics model only considers the coronary plaque wall in the model. The governing equation is much more complicated, including the motion equation, displacement-strain relationship, and constitutive equation of the coronary wall tissues, which can be solved to obtain the stress and strain conditions in the artery wall65. As part of the governing equations, the mechanical properties of coronary wall and plaque compositions described by constitutive equations have a profound effect on fully recapitulating the biomechanical conditions within the blood vessel34,66, which in turn play a crucial role in shaping plaque behaviors, like rupture and erosion67,68. 3) Lastly, fluid-structure interaction (FSI) model combines both fluid and solid models, and allows the simultaneous analysis of solid and fluid domains to derive the comprehensive biomechanical conditions in the coronary artery wall and blood flow69. Currently, most computational models of coronary plaques, especially solid and FSI models are created based on IVUS and OCT images as they can accurately reconstruct the vessel wall geometry with multiple plaque components70,71.
For computational models, the following essential elements need to be considered to accurately calculate biomechanical forces in the coronary plaque: a) coronary geometry reconstruction from patient-specific images, ; b) vessel and plaque component material properties; c) complete set of structure and/or flow governing equations (equation of motion, equation of continuity, structural stress/strain relationship, strain-displacement relationship); d) specified boundary conditions; e) initial conditions and residual stress/strain of the blood vessel69. Patient-specific coronary geometry was reconstructed by integrating IVUS or OCT images with angiography image to recover the vessel shape in three-dimensional space. However, this coronary geometry represents its in vivo state when the vessel is physiologically pressurized and longitudinally stretched69. Typically, an inverse process was conducted to find the zero-pressure geometry as the initial geometry for numerical simulation69,72. Under given boundary conditions, the blood would flow, and the vessel wall would start deforming in response to the external forces prescribed. Some computational studies even consider the coronary movement caused by cardiac contraction to accurately simulate the biomechanical conditions73. More details on the model construction process could be found in the previous comprehensive references46,54,69.
Role of biomechanical stresses in vulnerable plaque research
Mechanical factors in vulnerable plaque development and progression
Mechanical forces influence multiple aspects of vascular physiology and function, and are crucial in the pathological development of coronary atherosclerosis13,16. There are various types of atherosclerotic plaques, ranging from early lesions of atherosclerosis consisting of the adaptive lesion with fatty streak and fibrous tissue, to advanced complicated lesions16,19. Before the occurrence of critical behaviors like rupture and erosion, the plaques undergo a destabilization process, transforming into more advanced plaques with vulnerable features16. Computational studies have performed to uncover the complex relationship between the biomechanical factors and plaque progression.
In vitro experimental studies and clinical observations both reported that the plaque initiates at the location where low and oscillating shear stress usually occurs74,75. Many clinical studies have showed that the conclusion is also true for advanced plaque progression76. In the PREDICTION study with a large patient pool (n = 506), Stone et al. constructed CFD models based on IVUS image to demonstrate that low WSS (< 1.26 Pa) at baseline could predict plaque burden increase during 1-year follow-up period77. In another CFD study involving 20 patients with IVUS follow-up data, Samady et al. found that vessel cross-sections with baseline WSS below 1.0 Pa showed increased plaque area at follow-up (p = 0.027)78. Bourantas et al. further reported that low WSS and plaque burden were significant predictors of disease progression, defined as simultaneous lumen reduction and plaque burden increase79. They noted that combining WSS with plaque characteristics could achieve a prediction accuracy of around 85%, surpassing the use of plaque characteristics alone. Additionally, Liu et al. and other researchers have reached similar conclusions, showing that lesion sites with low WSS are linked to plaque growth80,81.
Besides shear stress, wall stress was also considered to aid in predicting plaque progression behaviors. By performing computational solid mechanics for 8182 virtual histology-IVUS frames from 101 plaque lesions, Costopoulos et al. found that areas with high wall stress was associated with larger plaque burden decrease at follow-up, compared to areas with low wall stress82. Another study by Wang et al, have demonstrated that integrating all morphological and biomechanical factors provided better results in predicting plaque progression83. Even though more prediction studies have echoed this finding, some researchers claimed that incorporating biomechanical factors into predictive models only yields marginal improvements in prediction accuracy (~ 5–10%)79,84,85. Furthermore, these prediction studies did not clearly show quantitative relationship between wall stress and plaque progression. Further mechanistic researches are warranted to validate these clinical observations via finding a proper animal model or other in vitro models for plaque rupture86,87.
Mechanical factors in plaque rupture
TCFA and plaque rupture
Pathological studies claimed that TCFAs were the precursor lesions prone to rupture18. However, large clinical studies, such as the PROSPECT and VIVA studies observed that only 5% of the identified TCFAs would undergo plaque rupture during a 3-year follow-up period, suggesting that plaque morphology alone cannot accurately predict future plaque rupture88,89. Biomechanical factors are considered as important cues triggering plaque rupture, so they might aid in identifying vulnerable plaques to rupture13.
Wall stress in plaque rupture
Mechanically specking, the determinants of plaque rupture lie in two aspects: intrinsic plaque vulnerability predisposes it to rupture, and extrinsic forces, such as biomechanical and hemodynamic stresses precipitate rupture17. When local wall stress within an atherosclerotic lesion exceeds its material strength, the plaque would disrupt34. These plaque disruptions are typically located in the fibrous cap or the shoulder region16, often co-localizing with the sites of maximum wall stress36. The material strength of the fibrous cap or shoulder region was determined by the integrated material strength and interactions of plaque compositions, such as collagen fiber, macrophage, and microcalcification in the plaque site67. Animal experimental studies have shown that excessive collagen breakdown combined with inadequate synthesis weakens plaques, thereby rendering these sites prone to rupture90,91. Computational studies also have provided extensive evidence to confirm the conclusion. The first finite element analysis of the plaque rupture was performed by Peterson et al92. based on histological images. The simulation results showed that high wall stress was found on the fibrous cap area, especially near the edge of the plaque cap, correlating well with the site of intimal tear at necropsy. This seminal study inspired the finite element models in studying the biomechanical mechanisms of plaque progression and rupture13. More quantitative analysis showed that the local maxima of wall stress normally exceeds 2250 mmHg ( ~ 300 kPa) for the ruptured plaques, a well-accepted threshold value for assessing plaque rupture risk from the biomechanical perspective36. It is worth noting in these studies using ex vivo histological images, that the post-rupture state of the plaque geometry from the histological section was repaired to reconstruct the pre-rupture geometry to investigate the biomechanical stress driving the rupture behavior36,92.
Computational studies based on in vivo images from patient-specific data also support that rupture risk was positively associated with the wall stress value93,94. In a comparison study with OCT images from 10 ACS patients and 10 stable patients, the wall stress values from the ruptured plaques were significantly higher than those from stable plaques93. The observation was also found in a similar study by performing 3D FSI models94. In vivo image based finite element analysis also established the impacts of multiple key morphological features on the stress calculation. As the fibrous cap thinner, the mechanical stress conditions would be higher, and the lipid component had a significant positive correlation with stress values95. All these analyses consistently conclude that higher wall stress is an important factor in triggering the rupture behavior of coronary plaques. Thus, biomechanical stress could therefore potentially act as a useful indicator for rupture risk assessment96. Besides, the mechanical stress would also influence the fibrous cap strength via mechanotransduction pathways13. The plaque areas experiencing high mechanical stress are often infiltrated by monocytes and macrophages to overexpress matrix metalloproteinases (MMPs)97. These MMPs are crucial in destabilizing the lesion though degradation and weakening of the collagenous extracellular matrix, posing a higher risk for plaque rupture98.
Wall shear stress in plaque rupture
Although the impact of wall stress on plaque rupture is rather established, the association between wall shear stress and rupture has not been studied extensively42. Many evidences have shown that the rupture occurs at the locations with elevated WSS99. In patients with ACS, it is observed that high shear stress is normally at the upstream plaque shoulder where rupture often occurs. Therefore, it has been postulated hat high shear stress might be the primary contributing factor100. In vivo evidence from a CFD analysis based on IVUS image from 20 patients with ruptured plaques, showed that local elevation of blood pressure and shear stress could be observed at the rupture sites on each plaque surface101. A possible mechanobiological explanation is that a observational studies reported high shear stress to activate MMPs and promote the fibrous cap thinning102 and eccentric remodeling103 in an arteriovenous fistula model. If a similar process occurs in advanced atherosclerotic lesions, it could explain the presence of a thin fibrous cap prone to rupture in high shear regions. Additionally, reduction in smooth muscle cell proliferation and increase in cell death were also reported at the local site with elevated WSS104. This would result in reduced extracellular matrix synthesis, responsible for potential plaque rupture.
Given both solid mechanics and hemodynamics greatly influenced the rupture of atherosclerotic plaques, a unifying theory was proposed by Pedrigi et al. that co-localization of high wall stress and shear stress-induced plaque weakening will finally lead to plaque rupture (Fig. 3(a))32. This hypothesized theory may explain the observation that only the minority of TCFAs lead to ACS. Still, further experimental and clinical imaging studies are needed to investigate the co-localization of these biomechanical parameters in identifying rupture-prone TCFAs.
Mechanical factors in plaque erosion
Compared to plaque rupture, plaque erosion is a recent emerging mechanism causing ACS. Therefore, fewer studies were performed on the mechanical factor inducing plaque erosion105. Wall shear stress, as the most studied mechanical factor, has been proposed as a driver of plaque erosion by promoting loss of endothelium. Early animal studies have yielded contradictory findings regarding the relationship between shear stress and the reduction of endothelial cells. Tricot et al. collected human carotid atherosclerotic plaques that exhibited endothelial apoptosis, as evidenced by immunostaining106. Their quantitative analysis showed a consistent preference for endothelial apoptosis in the downstream of the plaques, where low shear stress was supposed to be prevalent. Further animal studies have confirmed that the endothelial apoptosis would lead to the endothelial erosion and vessel thrombosis107. Rather quantifying the WSS magnitude, Sumi et al. qualitatively described that disturbed blood flow and oscillatory shear stress occurring after stenosis resulted in erosive damage and thrombus formation on the smooth muscle cell-rich neointima108. While these studies suggest a correlation between low WSS and endothelial cell apoptosis, a study using a rabbit model reported that increased WSS could lead to endothelial cell detachment and thrombus formation in areas rich in smooth muscle cells within atherosclerotic plaques109.
The inconsistent conclusion also persists in clinical studies in vivo by performing CFD analysis based on medical images (Fig. 3(b)). Table 1 lists the computational studies to investigate the mechanical driver of plaque erosion. Unfortunately, these studies also do not draw a firm conclusion105. In a case report by computing WSS from OCT based CFD analysis, Giannopoulos et al. demonstrated the co-localization of plaque erosion and low WSS in one patient involving ACS110. In contrast, Vergallo et al. reported a case where high WSS occurred at a location with future plaque erosion and luminal narrowing confirmed by OCT scan at 4-year follow-up111. Based on Table 1, the majority of CFD analysis reported that high WSS and WSS gradient values might the critical triggers for the onset of plaque erosion. Hakim et al. demonstrated that eroded plaques had significantly higher WSS and WSS gradient than stable ones112. Yamamoto et al. and M McElroy et al. also found that WSS and WSS gradient were higher at the plaque erosion sites compared to non-erosion sites for most of the patients examined26,113. Continued analysis showed that elevated WSS and WSS gradient at the erosion site persisted up to 12 months if no coronary stent was performed105. Except for the low and high WSS theories, one exceptional study by Campbell et al. indicated no association between high or low WSS values at plaque erosion sites114. The inconclusive of this study might be partially due to the small patient size (n = 3). Another reason may be that their coronary geometry was reconstructed from angiography by assuming an elliptical cross-section of vessel lumen. This assumption ignored some local coronary geometry variation, which great influenced local WSS values41. Even if most studies favor the high or low WSS theory, there are no large-scale studies with enough patient number to come to a definite conclusion with strong statistical power.
Wall stress and strain in plaque erosion
Due to the scarce of solid mechanical analysis, the relationship between plaque erosion and wall stress/strain has been rarely explored. To date, the sole study specifically addressing mechanical stress and strain for the onset of plaque erosion was conducted by Campbell and colleagues65. Their findings indicated no association between mechanical strain and plaque erosion, suggesting solid mechanics may not play a significant role in driving late-stage erosion-related complications.
Clinical perspectives
Integrative Perspective of Atherosclerotic Plaque Researches
Clinical diagnosis and management of coronary atherosclerotic diseases mainly relies on the plaque morphological characteristics. However, these morphological-information alone are not enough to provide accurate identification of culprit lesion for adverse coronary events13,89. For example, only 5% of the identified TCFAs are associated with ACS events caused by plaque rupture, implying that plaque morphology is not sufficient to predict plaque rupture13,88,89. As biomechanical factors are involved in plaque development, they might help to identify vulnerable plaques predispose to future coronary events. Therefore, it is necessary to integrating morphological and biomechanical factors for better understanding the plaque behaviors, including plaque rupture and erosion54,83.
This integrative approach was adopted in prior studies to reveal possible mechanical relationship with plaque progression, and destabilization process77,78, which would shed light on guiding the plaque risk assessment using these biomechanical stresses. The PREDICTION study found that low shear stress independently predicted luminal obstruction in patients with ACS77. Although clinical event rates were too low to sufficiently evaluate the effects of shear stress on clinical outcome77, the study suggests that biomechanical factors could be relevant in assessing progression-prone areas. In solid mechanics, a MRI-based study has proposed a scheme on carotid plaque biomechanical stress to classify the plaque vulnerability to rupture96. As data accumulated enough, the biomechanical wall stress hold the potential to rate the rupture risk of atherosclerotic plaques. Currently some threshold values of biomechanical stress have been proposed, however, detailed schemes on grading plaque vulnerability using these biomechanical factors have not been proposed yet based on in vivo image data36,96. This is also the case for plaque erosion. Given their close association, WSS may hold the potential for the risk stratification of future plaque erosion. And it can be imagined that the biomechanical factors would also integrate with morphological features for predicting the onset of the plaque erosion.
Machine learning or other artificial intelligence methods could also be adopted in this integrative approach to include more risk factors, not just morphological and biomechanical factors to assess future plaque behaviors, particularly when a large volume of data is being analyzed115,116,117. Based on 19826 patients with ACS, PRAISE study group have demonstrated the feasibility and effectiveness of machine learning-based approaches to predicting all-cause death, recurrent acute myocardial infarction, and major bleeding after acute coronary syndrome115. Koloi et al. also shown that machine learning methods can accurately predict early-stage coronary artery disease using a set of clinical characteristics and routine laboratory markers116. These reports demonstrate the strong capabilities of machine learning and artificial intelligence in predicting the behavior of atherosclerotic plaques, and applications of these methods should be recommended in future research.
Diagnostic and prognostic implications of image-based biomechanical models
Biomechanical modeling has been increasingly employed in the diagnosis, clinical decision-making, and treatment for patients with coronary artery disease115,118. A notable example is fractional flow reserve (FFR) in coronary atherosclerotic lesions, which reflects the pressure drop from the proximal aorta to the coronary segment distal to the lesion during maximal vasodilation118,119. According to the latest ESC guidelines, percutaneous coronary intervention (PCI) is recommended when FFR is ≤0.8120. Large clinical trials have shown that FFR-guided PCI achieves superior follow-up outcomes in coronary revascularization compared to angiogram-guided PCI121. CFD techniques based on coronary angiography have been demonstrated to be able to accurately calculate FFR in patients with stable angina, offering a less non-invasive alternative to catheter-based measurements for guiding PCI118. Another study also shown that CFD models can be used to simulate various potential surgical plans on treatment of intracranial aneurysms to determine the optimal one for a given patient122.
An important potential clinical application of solid mechanics would be identifying sites exposed to excessive forces associated with high plaque rupture risk34,36,96. Based on computational modeling, these studies have revealed a positive relationship between wall stress and plaque rupture risk36,95. Still, a diagnostic criterion based on wall stress to stratify the rupture risk should be established, and further should be extensively validated through large-scale clinical trials. With firm conclusion can be draw on the relationship between the plaque behaviors and these biomechanical stresses, the biomechanical indicators could revolutionize the triage and management of patients presenting with ACS13. Such an advancement would represent a step towards a more personalized approach, and illustrate the value of translation of basic science insights to clinical practice.
Biomechanical modulation in plaque rupture and erosion treatment
The biomechanics of plaque are not only relevant to the development of plaque rupture and erosion, but also should be considered when explaining the mechanistic role in their treatment. Effective clinical treatment option could be specifically explained by modulating the biomechanical conditions in the coronary plaques. Here are two simple examples. Biomechanical wall stress is a key factor in plaque rupture, as estimated by Laplace’s law38. Of utmost importance is to control the blood pressure to prevent rupture from high stress conditions for patients with ACS. Clinically, pressure-controlling medications like beta-blockers are typically recommended for these patients to reduce the coronary circumferential stress123. Another example is that elevated WSS and WSS gradient at non-stent treated erosion sites, persisting beyond one year, suggest a continued thrombogenic environment105. This suggests that WSS-related factors could be a therapeutic target modulated by the medication or surgery to change the local hemodynamic environment to help prevent future erosion, offering long-term benefits124.
Future Directions and Concluding Remarks
Mechanical mechanisms of plaque rupture and erosion
Understanding the mechanisms of plaque rupture and erosion are fundamental to develop tailored preventive strategy and treatment option13,125. Traditionally, our knowledge on their pathological mechanisms is mainly dependent on ex vivo autopsy studies. Recently, Intravascular OCT imaging technology is emerging as a powerful tool to visualize the distinctive morphological characteristics for reliably detecting these plaques51. This imaging modality not only provides a means to monitor the plaques in vivo, but also severs as a basis for constructing image-based computational models to simulate the biomechanical forces33. These forces are demonstrated to be important contributors for the development of these pathological processes30,34. Therefore, OCT-based computational modeling provides an efficient approach to uncover the mechanical mechanisms of these plaques13.
For plaque rupture, there is a general consensus among the experimental and computational studies that rupture usually occurs at the location with high circumferential wall stress38,98. Some further claimed that high shear stress also co-localizes with the rupture site101. However, mechanical forces are not the only determinant for rupture to happen17,126. The inflammation, fibrous cap microstructures and strength also influence the rupture behavior. And there is a still a long way to translate these biomechanical indicators into clinical setting.
Compared to plaque rupture, the biomechanical mechanisms of plaque erosion are far under-investigated. Even if most pathological, clinical and experimental animal studies observed that erosion preferentially occurs to the sites with turbulent flow127, they cannot reach an agreement whether low or high shear stress as the dominant mechanical cue to induce endothelium loss and superficial erosion105. Great efforts are need to advance our knowledge to the evolution of the plaque erosion. In order to reach a clear firm conclusion, several aspects should be carefully considered and evaluated to resolve the potential discrepancies in the existing literatures: a) There are some inconsistencies exist in these computational studies, like different image modality used for model construction (angiograph or OCT); different patient selection strategy (OCT scan from patients with future erosion, or patients already with erosion). These inconsistencies hindered the head-to-head comparison among these studies. Further efforts should be made to understand the differences among different situations and select the proper data set to investigate mechanisms related to plaque erosion; b) Large-scale computational studies with more patients are needed to identify robust mechanical factors triggering erosion with enough statistical power; c) More animal experiments or other approaches could be carried out to validate the biomechanical findings of plaque erosion studies. The latter two aspects were detailed in the following subsections. Once a clear, firm conclusion on the biomechanical trigger of erosion could be found, it would inspire prosperous molecular and cellular mechanistic studies for a deeper understanding of the mechanisms of erosion.
Large-scale studies for validation
To obtain a firm conclusion, large-scale studies with enough clinical data are need to reach a strong conclusion with high statistical power. Most of prior biomechanical studies typically had a sample size less than 50, especially for plaque erosion studies. The results of such studies are easily influenced by the patient variability, leading to inconclusive situation114. Large-scale studies are recommended to obtain convincing results on the mechanical mechanisms of plaque rupture and erosion in the future analysis.
Automation of computational simulation procedure for clinical application
Computational modeling approach is widely employed to simulate the biomechanical environment in the coronary plaques for possible mechanistic role of biomechanics in coronary atherosclerosis33. However, the biomechanical modeling process is typically time-consuming, especially when performing the FSI analysis69. Thus, one prerequisite for performing large-scale computational studies is to automate the computational simulation procedure to reduce labor cost and meet the requirements of early screening purposes. Currently, some advance in CFD analysis has been obtained, the computational solid mechanics model has made less progress partially due to the difficulty in generating the finite element mesh to incorporate the irregular shape of plaque components like lipid core or microcalcification69, and solving more complex governing equations. There are multiple components in the atherosclerotic plaques, and it is difficult to obtain their mechanical properties for computational modeling, especially for patient-specific modeling. Furthermore, quantifying the precise boundary conditions for computational solid models, like the supporting forces from the perivascular connective tissue is also challenging. More attempts should be made to overcome these issues if we would like to explore more impacts of solid mechanics in plaque behaviors.
In vivo animal models or in vitro tissue engineering model of coronary atherosclerosis
It is well-recognized that the atherosclerotic plaques in the animal models are different from the human ones128. Even though much progress has been made to create animal models for plaque rupture and erosion, they have limited ability in fully mimicking the natural history of rupture and erosive behavior of plaques86,127,129. These challenges pose much difficulty to study the mechanisms to the pathology of these plaque lesions. Another choice is 2D cellular culture model. Given they cannot recapitulate the complex vascular structure, with multiple vascular cells, extracellular matrix, and other substrates in the coronary artery, 2D cellular culture models are often employed to investigate the cellular and molecular mechanisms in atherosclerosis130. Recently, Tissue engineering approach has emerged as a valuable in vitro model for studying atherosclerosis130. Tissue engineering blood vessel with early atherosclerosis was created, which showed promise to replace the animal models for studying atherosclerotic process131. A prototype work has already performed to understand the biomechanical impact of microcalcification on rupture132. This approach has advantages over 2D cellular culture model by incorporating the complex interactions between vascular cells and substrate matrix130. And it would be easier to control the mechanical environments in the tissue-engineered blood vessel by controlling flow rate or modulating engineered vessel matrix stiffness133. Therefore, tissue-engineered approach may hold the potential to validate key mechanical factors that predispose to erosion. Additionally, this approach enables detailed observation of plaque behaviors at the micro-level134, like endothelial denudation, which are challenging to detect in clinical trials and animal studies. Nevertheless, this novel bioengineering approach is still in its infancy and requires more investigations.
To conclude, biomechanical stresses play a crucial role in influencing endothelial function, vascular remodeling, plaque development and deleterious behaviors causing ACS. Due to the challenges of directly measuring these stresses in human coronary arteries, computational biomechanical models have become the mainstream approaches to obtain these mechanical data, and should be incorporated with morphological characteristics to investigate the mechanisms to plaque rupture and erosion. High-resolution OCT imagebased finite element models have revealed a significant difference in the biomechanical environments predisposing to fibrous cap rupture or superficial erosion. A deeper understanding of the effects of mechanical forces in these plaque behaviors will further the developments of novel drugs, enhancing diagnostic tools and informing clinical decision-making for tailored management of plaque rupture and erosion.
Data availability
No datasets were generated or analysed during the current study.
Change history
17 June 2025
A Correction to this paper has been published: https://doi.org/10.1038/s44325-025-00069-3
References
Timmis, A. et al. Global epidemiology of acute coronary syndromes. Nat Rev Cardiol, https://doi.org/10.1038/s41569-023-00884-0 (2023).
Eisen, A., Giugliano, R. P. & Braunwald, E. Updates on Acute Coronary Syndrome: A Review. JAMA Cardiol. 1, 718–730, (2016).
Byrne, R. A. et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur. Heart J. 44, 3720–3826 (2023).
Bhatt, D. L., Lopes, R. D. & Harrington, R. A. Diagnosis and Treatment of Acute Coronary Syndromes: A Review. JAMA 327, 662–675, (2022).
Kumar, A. & Cannon, C. P. Acute coronary syndromes: diagnosis and management, part I. Mayo Clin. Proc. 84, 917–938 (2009).
Yuan, D. et al. New Concepts on the Pathophysiology of Acute Coronary Syndrome. Rev. Cardiovasc. Med. 24, 112 (2023).
Waterbury, T. M. et al. Non-atherosclerotic causes of acute coronary syndromes. Nat. Rev. Cardiol. 17, 229–241 (2020).
Saw, J., Mancini, G. B. J. & Humphries, K. H. Contemporary Review on Spontaneous Coronary Artery Dissection. J. Am. Coll. Cardiol. 68, 297–312 (2016).
Kanwar, S. S. et al. Acute coronary syndromes without coronary plaque rupture. Nat. Rev. Cardiol. 13, 257–265 (2016).
Virmani, R., Burke, A. P., Farb, A. & Kolodgie, F. D. Pathology of the vulnerable plaque. J. Am. Coll. Cardiol. 47, C13–18, (2006).
Meteva, D., Seppelt, C., Abdelwahed, Y. S. & Leistner, D. M. Distinct pathological mechanisms distinguish acute coronary syndrome caused by plaque erosion from plaque rupture. Curr. Opin. Cardiol. 36, 793–797 (2021).
Furie, B. & Furie, B. C. Mechanisms of thrombus formation. N. Engl. J. Med 359, 938–949 (2008).
Kwak, B. R. et al. Biomechanical factors in atherosclerosis: mechanisms and clinical implications. Eur. heart J. 35, 3013–3020 (2014).
Libby, P., Pasterkamp, G., Crea, F. & Jang, I. K. Reassessing the Mechanisms of Acute Coronary Syndromes. Circ. Res 124, 150–160 (2019).
Bentzon, J. F., Otsuka, F., Virmani, R. & Falk, E. Mechanisms of plaque formation and rupture. Circ. Res 114, 1852–1866 (2014).
Virmani, R., Kolodgie, F. D., Burke, A. P., Farb, A. & Schwartz, S. M. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb. Vasc. Biol. 20, 1262–1275 (2000).
Falk, E., Shah, P. K. & Fuster, V. Coronary plaque disruption. Circulation 92, 657–671 (1995).
Kolodgie, F. D. et al. The thin-cap fibroatheroma: a type of vulnerable plaque: the major precursor lesion to acute coronary syndromes. Curr. Opin. Cardiol. 16, 285–292 (2001).
Stary, H. C. et al. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation 92, 1355–1374 (1995).
van der Wal, A. C., Becker, A. E., van der Loos, C. M. & Das, P. K. Site of intimal rupture or erosion of thrombosed coronary atherosclerotic plaques is characterized by an inflammatory process irrespective of the dominant plaque morphology. Circulation 89, 36–44 (1994).
Arbustini, E. et al. Plaque erosion is a major substrate for coronary thrombosis in acute myocardial infarction. Heart (Br. Card. Soc.) 82, 269–272 (1999).
Virmani, R., Burke, A. P. & Farb, A. Plaque rupture and plaque erosion. Thromb. Haemost. 82, 1–3 (1999).
Farb, A. et al. Coronary plaque erosion without rupture into a lipid core. A frequent cause of coronary thrombosis in sudden coronary death. Circulation 93, 1354–1363 (1996).
Sun, J. et al. Spatial Transcriptional Mapping Reveals Site-Specific Pathways Underlying Human Atherosclerotic Plaque Rupture. J. Am. Coll. Cardiol. 81, 2213–2227 (2023).
Petrossian, G. et al. Role of Intracoronary Imaging in Acute Coronary Syndromes. US Cardiology Review 16, https://doi.org/10.15420/usc.2022.03 (2022).
Yamamoto, E. et al. Endothelial Shear Stress and Plaque Erosion. A Computat. Fluid Dyn. Optical Coherence Tomogr. Study JACC Cardiovasc Imaging 12, 374–375 (2019).
Baaten, C., Nagy, M., Bergmeier, W., Spronk, H. M. H. & van der Meijden, P. E. J. Platelet biology and function: plaque erosion vs. rupture. Eur. Heart J. 45, 18–31 (2024).
Dai, J. et al. In vivo predictors of plaque erosion in patients with ST-segment elevation myocardial infarction: a clinical, angiographical, and intravascular optical coherence tomography study. Eur. Heart J. 39, 2077–2085 (2018).
Sato, Y. et al. Proportion of fibrin and platelets differs in thrombi on ruptured and eroded coronary atherosclerotic plaques in humans. Heart (Br. Card. Soc.) 91, 526–530 (2005).
Gregory, F. Role of mechanical stress and neutrophils in the pathogenesis of plaque erosion. Atherosclerosis 318, 60–69 (2021).
Andreou, I. et al. How do we prevent the vulnerable atherosclerotic plaque from rupturing? Insights from in vivo assessments of plaque, vascular remodeling, and local endothelial shear stress. J. Cardiovasc. Pharmacol. therapeutics 20, 261–275 (2015).
Pedrigi, R. M. et al. Thin-cap fibroatheroma rupture is associated with a fine interplay of shear and wall stress. Arteriosclerosis, thrombosis, Vasc. Biol. 34, 2224–2231 (2014).
Thondapu, V. et al. Biomechanical stress in coronary atherosclerosis: emerging insights from computational modelling. Eur. heart J. 38, 81–92 (2017).
Brown, A. J. et al. Role of biomechanical forces in the natural history of coronary atherosclerosis. Nat. Rev. Cardiol. 13, 210–220 (2016).
Cameron, J. N. et al. Exploring the relationship between biomechanical stresses and coronary atherosclerosis. Atherosclerosis 302, 43–51 (2020).
Cheng, G. C., Loree, H. M., Kamm, R. D., Fishbein, M. C. & Lee, R. T. Distribution of circumferential stress in ruptured and stable atherosclerotic lesions. A structural analysis with histopathological correlation. Circulation 87, 1179–1187 (1993).
Ohayon, J., Teppaz, P., Finet, G. & Rioufol, G. In-vivo prediction of human coronary plaque rupture location using intravascular ultrasound and the finite element method. Coron. Artery Dis. 12, 655–663 (2001).
Arroyo, L. H. & Lee, R. T. Mechanisms of plaque rupture: mechanical and biologic interactions. Cardiovascular Res. 41, 369–375 (1999).
Fung, Y. C. & Liu, S. Q. Strain distribution in small blood vessels with zero-stress state taken into consideration. Am. J. Physiol. 262, H544–552, (1992).
Ohayon, J. et al. Influence of residual stress/strain on the biomechanical stability of vulnerable coronary plaques: potential impact for evaluating the risk of plaque rupture. Am. J. Physiol. Heart Circulat. Physiol. 293, H1987–1996, (2007).
Gijsen, F. et al. Expert recommendations on the assessment of wall shear stress in human coronary arteries: existing methodologies, technical considerations, and clinical applications. Eur. Heart J. 40, 3421–3433 (2019).
Katritsis, D. G., Pantos, J. & Efstathopoulos, E. Hemodynamic factors and atheromatic plaque rupture in the coronary arteries: from vulnerable plaque to vulnerable coronary segment. Coron. artery Dis. 18, 229–237 (2007).
Malek, A. M., Alper, S. L. & Izumo, S. Hemodynamic shear stress and its role in atherosclerosis. JAMA 282, 2035–2042, (1999).
Asakura, T. & Karino, T. Flow patterns and spatial distribution of atherosclerotic lesions in human coronary arteries. Circ. Res 66, 1045–1066 (1990).
Ekmejian, A. A. et al. Advances in the Computational Assessment of Disturbed Coronary Flow and Wall Shear Stress: A Contemporary Review. J. Am. Heart Assoc. 13, e037129 (2024).
Carpenter, H. J., Gholipour, A., Ghayesh, M. H., Zander, A. C. & Psaltis, P. J. A review on the biomechanics of coronary arteries. Int. J. Engineer. Sci. 147, https://doi.org/10.1016/j.ijengsci.2019.103201 (2020).
Gholipour, A., Ghayesh, M. H., Zander, A. C. & Psaltis, P. J. In vivo based biomechanics of right and left coronary arteries. Int. J. Engineer. Sci. 154, https://doi.org/10.1016/j.ijengsci.2020.103281 (2020).
Burke, A. P. et al. Coronary risk factors and plaque morphology in men with coronary disease who died suddenly. N. Engl. J. Med. 336, 1276–1282 (1997).
Gutstein, D. E. & Fuster, V. Pathophysiology and clinical significance of atherosclerotic plaque rupture. Cardiovasc Res 41, 323–333 (1999).
Sugane, H. et al. Cardiac outcomes in patients with acute coronary syndrome attributable to calcified nodule. Atherosclerosis 318, 70–75 (2021).
Jia, H. et al. In vivo diagnosis of plaque erosion and calcified nodule in patients with acute coronary syndrome by intravascular optical coherence tomography. J. Am. Coll. Cardiol. 62, 1748–1758 (2013).
Ozaki, Y. et al. Coronary CT angiographic characteristics of culprit lesions in acute coronary syndromes not related to plaque rupture as defined by optical coherence tomography and angioscopy. Eur. Heart J. 32, 2814–2823 (2011).
Tarkin, J. M. et al. Imaging Atherosclerosis. Circ. Res 118, 750–769 (2016).
Lv, R. et al. Image-based biomechanical modeling for coronary atherosclerotic plaque progression and vulnerability prediction. Int J. Cardiol. 352, 1–8 (2022).
Jang, I. K. et al. Visualization of coronary atherosclerotic plaques in patients using optical coherence tomography: comparison with intravascular ultrasound. J. Am. Coll. Cardiol. 39, 604–609 (2002).
Jang, I. K. et al. In vivo characterization of coronary atherosclerotic plaque by use of optical coherence tomography. Circulation 111, 1551–1555 (2005).
Tearney, G. J. et al. Consensus Standards for Acquisition, Measurement, and Reporting of Intravascular Optical Coherence Tomography Studies. J. Am. Coll. Cardiol. 59, 1058–1072 (2012).
Collet, C. et al. Reviewing imaging modalities for the assessment of plaque erosion. Atherosclerosis 318, 52–59 (2021).
Wang, G. et al. Diagnostic Performance of 60 MHz High-Definition Intravascular Ultrasound versus Fourier Domain Optical Coherence Tomography for Identifying Plaque Rupture, Plaque Erosion, and Thrombosis in a Rabbit Model. Rev. Cardiovasc Med 24, 76 (2023).
Higuma, T. et al. A Combined Optical Coherence Tomography and Intravascular Ultrasound Study on Plaque Rupture, Plaque Erosion, and Calcified Nodule in Patients With ST-Segment Elevation Myocardial Infarction. Incid. Morphologic Charact. Outcomes Percutaneous Coron. Intervention. Jacc. Cardiovasc. intervent. 8, 1166–1176 (2015).
Tian, J. et al. Distinct morphological features of ruptured culprit plaque for acute coronary events compared to those with silent rupture and thin-cap fibroatheroma: a combined optical coherence tomography and intravascular ultrasound study. J. Am. Coll. Cardiol. 63, 2209–2216 (2014).
Kolte, D. et al. Optical Coherence Tomography of Plaque Erosion: JACC Focus Seminar Part 2/3. J. Am. Coll. Cardiol. 78, 1266–1274 (2021).
Thondapu, V. et al. High spatial endothelial shear stress gradient independently predicts site of acute coronary plaque rupture and erosion. Cardiovasc Res 117, 1974–1985 (2021).
Russo, G. et al. Coronary artery plaque rupture and erosion: Role of wall shear stress profiling and biological patterns in acute coronary syndromes. Int J. Cardiol. 370, 356–365 (2023).
Campbell, I. C. et al. Biomechanics and inflammation in atherosclerotic plaque erosion and plaque rupture: implications for cardiovascular events in women. PLoS One 9, e111785 (2014).
Tang, D. et al. Quantifying effects of plaque structure and material properties on stress distributions in human atherosclerotic plaques using 3D FSI models. J. Biomech. Eng. 127, 1185–1194 (2005).
Jansen, I. et al. The interplay of collagen, macrophages, and microcalcification in atherosclerotic plaque cap rupture mechanics. Basic Res Cardiol. 119, 193–213 (2024).
Partida, R. A., Libby, P., Crea, F. & Jang, I. K. Plaque erosion: a new in vivo diagnosis and a potential major shift in the management of patients with acute coronary syndromes. Eur. Heart J. 39, 2070–2076 (2018).
Tang, D. et al. Image-based modeling for better understanding and assessment of atherosclerotic plaque progression and vulnerability: data, modeling, validation, uncertainty and predictions. J. Biomech. 47, 834–846 (2014).
Wang, J. et al. Optical coherence tomography-based patient-specific coronary artery reconstruction and fluid-structure interaction simulation. Biomech. Model Mechanobiol. 19, 7–20 (2020).
Yang, C. et al. In vivo IVUS-based 3-D fluid-structure interaction models with cyclic bending and anisotropic vessel properties for human atherosclerotic coronary plaque mechanical analysis. IEEE Trans. Biomed. Eng. 56, 2420–2428 (2009).
Speelman, L. et al. Initial stress in biomechanical models of atherosclerotic plaques. J. Biomech. 44, 2376–2382 (2011).
Wang, J. et al. Impact of cyclic bending on coronary hemodynamics. Biomech. Model Mechanobiol. 22, 729–738 (2023).
Ku, D. N., Giddens, D. P., Zarins, C. K. & Glagov, S. Pulsatile flow and atherosclerosis in the human carotid bifurcation. Positive correlation between plaque location and low oscillating shear stress. Arteriosclerosis 5, 293–302 (1985).
Cecchi, E. et al. Role of hemodynamic shear stress in cardiovascular disease. Atherosclerosis 214, 249–256 (2011).
Wentzel, J. J. et al. Endothelial shear stress in the evolution of coronary atherosclerotic plaque and vascular remodelling: current understanding and remaining questions. Cardiovasc. Res. 96, 234–243 (2012).
Stone, P. H. et al. Prediction of progression of coronary artery disease and clinical outcomes using vascular profiling of endothelial shear stress and arterial plaque characteristics: the PREDICTION Study. Circulation 126, 172–181 (2012).
Samady, H. et al. Coronary artery wall shear stress is associated with progression and transformation of atherosclerotic plaque and arterial remodeling in patients with coronary artery disease. Circulation 124, 779–788 (2011).
Bourantas, C. V. et al. Utility of Multimodality Intravascular Imaging and the Local Hemodynamic Forces to Predict Atherosclerotic Disease Progression. JACC Cardiovasc Imaging 13, 1021–1032 (2020).
Corban, M. T. et al. Combination of plaque burden, wall shear stress, and plaque phenotype has incremental value for prediction of coronary atherosclerotic plaque progression and vulnerability. Atherosclerosis 232, 271–276 (2014).
Liu, X. et al. Prediction of coronary plaque progression using biomechanical factors and vascular characteristics based on computed tomography angiography. Computer Assist. Surg. (Abingdon, Engl.) 22, 286–294 (2017).
Costopoulos, C. et al. Heterogeneity of Plaque Structural Stress Is Increased in Plaques Leading to MACE. Insights PROSPECT Study Jacc. Cardiovasc. imaging 13, 1206–1218 (2020).
Wang, L. et al. Fluid-structure interaction models based on patient-specific IVUS at baseline and follow-up for prediction of coronary plaque progression by morphological and biomechanical factors: A preliminary study. J. Biomech. 68, 43–50 (2018).
Sakellarios, A. et al. Prediction of atherosclerotic disease progression using LDL transport modelling: a serial computed tomographic coronary angiographic study. Eur. Heart J. Cardiovasc. Imaging 18, 11–18 (2017).
Lee, J. M. et al. Identification of High-Risk Plaques Destined to Cause Acute Coronary Syndrome Using Coronary Computed Tomographic Angiography and Computational Fluid Dynamics. JACC Cardiovasc. Imaging 12, 1032–1043 (2019).
Cullen, P. et al. Rupture of the atherosclerotic plaque: does a good animal model exist? Arterioscler Thromb. Vasc. Biol. 23, 535–542 (2003).
van der Heiden, K., Hoogendoorn, A., Daemen, M. J. & Gijsen, F. J. Animal models for plaque rupture: a biomechanical assessment. Thrombosis Haemost. 115, 501–508 (2016).
Calvert, P. A. et al. Association between IVUS findings and adverse outcomes in patients with coronary artery disease. VIVA (VH-IVUS Vulnerable Atherosclerosis) Study JACC Cardiovasc. Imaging 4, 894–901 (2011).
Stone, G. W. et al. A prospective natural-history study of coronary atherosclerosis. N. Engl. J. Med 364, 226–235 (2011).
Rekhter, M. D. et al. Hypercholesterolemia causes mechanical weakening of rabbit atheroma : local collagen loss as a prerequisite of plaque rupture. Circ. Res 86, 101–108 (2000).
Rekhter, M. D. et al. Animal model that mimics atherosclerotic plaque rupture. Circ. Res 83, 705–713 (1998).
Richardson, P. D., Davies, M. J. & Born, G. V. Influence of plaque configuration and stress distribution on fissuring of coronary atherosclerotic plaques. Lancet 2, 941–944 (1989).
Milzi, A. et al. Coronary plaque composition influences biomechanical stress and predicts plaque rupture in a morpho-mechanic OCT analysis. eLife 10, https://doi.org/10.7554/eLife.64020 (2021).
Zhao, C. et al. Plaque Ruptures Are Related to High Plaque Stress and Strain Conditions: Direct Verification by Using In Vivo OCT Rupture Data and FSI Models. Arteriosclerosis thrombosis, Vasc. Biol. 44, 1617–1627 (2024).
Wang, L. et al. Morphological and Stress Vulnerability Indices for Human Coronary Plaques and Their Correlations with Cap Thickness and Lipid Percent: An IVUS-Based Fluid-Structure Interaction Multi-patient Study. PLoS Comput. Biol. 11, e1004652 (2015).
Gijsen, F. J. et al. Carotid Plaque Morphological Classification Compared With Biomechanical Cap Stress: Implications for a Magnetic Resonance Imaging-Based Assessment. Stroke 46, 2124–2128 (2015).
Yamamoto, K., Ikeda, U. & Shimada, K. Role of mechanical stress in monocytes/macrophages: implications for atherosclerosis. Curr. Vasc. Pharmacol. 1, 315–319 (2003).
Lee, R. T., Schoen, F. J., Loree, H. M., Lark, M. W. & Libby, P. Circumferential stress and matrix metalloproteinase 1 in human coronary atherosclerosis. Implications for plaque rupture. Arteriosclerosis thrombosis, Vasc. Biol. 16, 1070–1073 (1996).
Gertz, S. D. & Roberts, W. C. Hemodynamic shear force in rupture of coronary arterial atherosclerotic plaques. Am. J. Cardiol. 66, 1368–1372 (1990).
Slager, C. J. et al. The role of shear stress in the destabilization of vulnerable plaques and related therapeutic implications. Nat. Clin. Pract. Cardiovasc. Med. 2, 456–464 (2005).
Fukumoto, Y. et al. Localized elevation of shear stress is related to coronary plaque rupture: a 3-dimensional intravascular ultrasound study with in-vivo color mapping of shear stress distribution. J. Am. Coll. Cardiol. 51, 645–650 (2008).
Mattsson, E. J., Kohler, T. R., Vergel, S. M. & Clowes, A. W. Increased blood flow induces regression of intimal hyperplasia. Arterioscler Thromb. Vasc. Biol. 17, 2245–2249 (1997).
Tronc, F. et al. Role of matrix metalloproteinases in blood flow-induced arterial enlargement: interaction with NO. Arterioscler Thromb. Vasc. Biol. 20, E120–E126 (2000).
Clowes, A. W. & Berceli, S. A. Mechanisms of vascular atrophy and fibrous cap disruption. Ann. N. Y Acad. Sci. 902, 153–161 (2000). discussion 161-152.
Kim, H. O. et al. High endothelial shear stress and stress gradient at plaque erosion persist up to 12 months. Int J. Cardiol. 357, 1–7 (2022).
Tricot, O. et al. Relation between endothelial cell apoptosis and blood flow direction in human atherosclerotic plaques. Circulation 101, 2450–2453 (2000).
Durand, E. et al. In vivo induction of endothelial apoptosis leads to vessel thrombosis and endothelial denudation: a clue to the understanding of the mechanisms of thrombotic plaque erosion. Circulation 109, 2503–2506 (2004).
Sumi, T. et al. Disturbed blood flow induces erosive injury to smooth muscle cell-rich neointima and promotes thrombus formation in rabbit femoral arteries. J. Thromb. Haemost. 8, 1394–1402 (2010).
Sameshima, N. et al. The values of wall shear stress, turbulence kinetic energy and blood pressure gradient are associated with atherosclerotic plaque erosion in rabbits. J. Atheroscler. Thromb. 21, 831–838 (2014).
Giannopoulos, A. A., Antoniadis, A. P., Croce, K. & Chatzizisis, Y. S. Erosion of Thin-Cap Fibroatheroma in an Area of Low Endothelial Shear Stress. Anat. Local Hemodyna. Environ. Dictate Outcomes JACC Cardiovasc Inter. 9, e77–e78 (2016).
Vergallo, R. et al. Coronary plaque erosion developing in an area of high endothelial shear stress: insights from serial optical coherence tomography imaging. Coron. artery Dis. 30, 74–75 (2019).
Hakim, D. et al. The role of endothelial shear stress, shear stress gradient, and plaque topography in plaque erosion. Atherosclerosis 376, 11–18 (2023).
McElroy, M. et al. Identification of the haemodynamic environment permissive for plaque erosion. Sci. Rep. 11, 7253 (2021).
Campbell, I. C. et al. Computational Fluid Dynamics Simulations of Hemodynamics in Plaque Erosion. Cardiovasc. Eng Technol. 4, https://doi.org/10.1007/s13239-013-0165-3 (2013).
D’Ascenzo, F. et al. Machine learning-based prediction of adverse events following an acute coronary syndrome (PRAISE): a modelling study of pooled datasets. Lancet 397, 199–207 (2021).
Koloi, A. et al. Predicting early-stage coronary artery disease using machine learning and routine clinical biomarkers improved by augmented virtual data. Eur. Heart J. Digit Health 5, 542–550 (2024).
Lv, R. et al. Combining IVUS + OCT Data, Biomechanical Models and Machine Learning Method for Accurate Coronary Plaque Morphology Quantification and Cap Thickness and Stress/Strain Index Predictions. J. Funct. Biomater 14, https://doi.org/10.3390/jfb14010041 (2023).
Tu, S. et al. Diagnostic Accuracy of Fast Computational Approaches to Derive Fractional Flow Reserve From Diagnostic Coronary Angiography. Int. Multicent. FAVOR Pilot Study JACC Cardiovasc. Inter. 9, 2024–2035 (2016).
Pijls, N. H. et al. Measurement of fractional flow reserve to assess the functional severity of coronary-artery stenoses. N. Engl. J. Med 334, 1703–1708 (1996).
Neumann, F. J. et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 40, 87–165 (2019).
Tonino, P. A. et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N. Engl. J. Med 360, 213–224 (2009).
Sarrami-Foroushani, A. et al. In-silico trial of intracranial flow diverters replicates and expands insights from conventional clinical trials. Nat. Commun. 12, 3861 (2021).
Williams, M. J., Low, C. J., Wilkins, G. T. & Stewart, R. A. Randomised comparison of the effects of nicardipine and esmolol on coronary artery wall stress: implications for the risk of plaque rupture. Heart 84, 377–382 (2000).
Cheng, H. et al. Effects of shear stress on vascular endothelial functions in atherosclerosis and potential therapeutic approaches. Biomed. Pharmacother. 158, 114198 (2023).
Libby, P. & Pasterkamp, G. Requiem for the ‘vulnerable plaque. Eur. Heart J. 36, 2984–2987 (2015).
Zaman, A. G., Helft, G., Worthley, S. G. & Badimon, J. J. The role of plaque rupture and thrombosis in coronary artery disease. Atherosclerosis 149, 251–266 (2000).
Franck, G. et al. Flow Perturbation Mediates Neutrophil Recruitment and Potentiates Endothelial Injury via TLR2 in Mice: Implications for Superficial Erosion. Circ. Res 121, 31–42 (2017).
Getz, G. S. & Reardon, C. A. Animal models of atherosclerosis. Arterioscler Thromb. Vasc. Biol. 32, 1104–1115 (2012).
Quillard, T., Franck, G., Mawson, T., Folco, E. & Libby, P. Mechanisms of erosion of atherosclerotic plaques. Curr. Opin. Lipido. 28, 434–441 (2017).
Chen, J. et al. Recent Progress in in vitro Models for Atherosclerosis Studies. Front. Cardiovasc. Med 8, 790529 (2021).
Lee, J. H. et al. Emulating Early Atherosclerosis in a Vascular Microphysiological System Using Branched Tissue-Engineered Blood Vessels. Adv. Biol. (Weinh.) 5, e2000428 (2021).
Jansen, I. et al. A tissue-engineered model of the atherosclerotic plaque cap: Toward understanding the role of microcalcifications in plaque rupture. APL Bioeng. 7, 036120 (2023).
Wang, L. et al. A new approach of using organ-on-a-chip and fluid-structure interaction modeling to investigate biomechanical characteristics in tissue-engineered blood vessels. Front. Physiol. 14, 1210826 (2023).
Chen, Z. et al. Real-time observation of leukocyte-endothelium interactions in tissue-engineered blood vessel. Lab Chip 18, 2047–2054 (2018).
Acknowledgements
This study was funded by Key R&D project of Heilongjiang Province (grant number 2022ZX06C07 to H.J.), National Natural Science Foundation of China (grant number 81722025 to H.J.; grant number 11972117 to D.T.; grant number 11802060 to L.W.), Basic Research Plan Natural Science Foundation of Jiangsu Province (grant number BK20232023 to L.W.), Natural Science Foundation of Shandong Province (grant number ZR2024QA110 to R.L.). The funder played no role in study design, data collection, analysis and interpretation of data, or the writing of this manuscript.
Author information
Authors and Affiliations
Contributions
L.W., Y.Z., C.Z., A.M., R.L., M.Y., X.G., G.S.M., D.T., H.J., and B.Y. contributed to the study conception and design and edited the manuscript. L.W. wrote the first draft of the manuscript. All authors read, reviewed, and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
Author Haibo Jia is a Guest Editor at npj Cardiovascular Health. Haibo Jia was not involved in the journal’s review of, or decisions related to, this manuscript.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, L., Zhu, Y., Zhao, C. et al. Role of biomechanical factors in plaque rupture and erosion: insight from intravascular imaging based computational modeling. npj Cardiovasc Health 2, 12 (2025). https://doi.org/10.1038/s44325-025-00048-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s44325-025-00048-8
This article is cited by
-
Advancing global cardiovascular research and clinical translation
npj Cardiovascular Health (2025)