Abstract
The squirting cucumber (Ecballium elaterium) exhibits unique mechanical adaptations for seed dispersal. When ripe, the fruit explodes due to turgor pressure, ejecting seeds and fluid several meters away. Early 1900s studies examined this phenomenon, but modern techniques like 3D imaging, electron microscopy, force measurement, and high-speed video offer new insights. We investigated the plant’s morphological changes during ripening using micro-CT and calculated seed ejection speed and distance with high-speed videos. The fruit stem straightens and the angle to the fruit increases to 53° upon ripening. Seeds, arranged in six longitudinal rows, reach speeds of approximately 10 m/s and distances up to 12 m. The fluid ejected with seeds likely reduces friction, and the seed envelope forms a mucilaginous coat that becomes a strong adhesive when dried, with forces up to 27.5 N. These findings enhance our understanding of this plant’s seed dispersal mechanisms and adaptations for seed establishment and germination.
Similar content being viewed by others
Introduction
Plants utilize many different mechanisms for seed dispersal, such as (1) allochory, the dispersal through an external vector, for example the dispersal by animals, water or wind and (2) autochory, the self-dispersal, with gravity or ballistic actions (ballochory) as specific examples of autochory1,2,3. In the case of biological shooting mechanisms, the shooting in plants and fungi can, for example, be facilitated through osmosis or by energy storage with elastic tissues or dedicated catapult mechanisms4. In detail, plants can explode fruit cases, to disperse their seeds into the surroundings several meters away from the plant5. One exciting, ‘explosive’ example for ballochory is the fruit of Ecballium elaterium (Cucurbitaceae).
The ‘squirting cucumber’, E. elaterium (see ref. 6 for basic fruit characteristics), is native to the Mediterranean region and really lives up to its name. As soon as the fruits of the plant ripen, they ‘explode’ and eject their seeds up to the distance of several meters7,8,9. The ‘explosion’ is triggered by a turgor mechanism, which depends on the rising internal pressure of the liquid within the fruit and an interplay of rising turgors in the inner parenchyma and the resistance tissue8,10,11,12. The fruit detaches from the plant at a predetermined breaking point on the stem and ejects its seeds from this opening over the canopy of its own and neighboring plants. This mechanism is quick and forceful and that is why resembles an explosion, as some of the seeds are ejected at speeds of between 4 and 15 m/s and distances of approximately up to 13 m7,13,14.
The 3–5 cm long, ovoid fruits of E. elaterium have a pericarp with soft, juicy spikes and grow about 15–25 cm above the ground with one fruit per vertically standing stem7,13. Thereby, the fruits grow to finally reach an angle of approximately 55° to the stem and thus achieve a shooting angle of 53–55° while the stem straightens7,12,15. Inside the fruit, the mostly dark brown seeds are accumulated at the hilum and are arranged on vascular strands with varying numbers of seeds12,16. These vascular bundles form a three-paired plate and meet at the median line in the middle of the fruit12,17. Here the seeds are arranged in six longitudinal rows with 8–10 seeds per row and accumulate to 53 seeds per fruit17,18. However, the number of seeds per fruit varies greatly, ranging from 3 to 53 seeds, depending on environmental conditions and plant health18,19,20. After fruit explosion, the seed coat forms a gel-like envelope around the seed, in which cellulose fibers unfold from the seed coat (epidermal cells of the secondary cell wall) and spread out, as soon as the seed gets in contact with water16,21.
Over the last 150 years, many scientist have studied the structure and dispersal mechanism of E. elaterium and reported about the inner arrangement of opened fruits, shooting angles and seed dispersal speed and distance9,12,13,14,17,22. However, many aspects of this fascinating plant have not been completely understood and modern technology allows for more detailed insights, as a result of which some studies have recently been carried out on the squirting cucumber12,23. The ballistic mechanism has been used as inspiration for biomimetic hydrogel launchers23, however, details in the understanding of the natural mechanism are still incomplete. Nowadays, with numerous modern imaging and experimental techniques, we have the chance to study the dispersal mechanism of E. elaterium in further detail to find out more about the seed arrangement in the fruit and its orientation by leaving the fruit, just as the resulting dispersal speed and direction. Moreover, the morphological changes of fruit and stem over the ripening process were of interest, additionally to the seed structure and its mechanical properties, which might aid in the dispersal. In the present paper, we focused on (1) the 3D arrangement of the seeds inside the fruit using µCT tomography, (2) morphological changes of the plant stems during the ripening process, (3) determination of speed of seed ejection and possible dispersal distances using high-speed video recordings. Additionally, (4) the ability of seeds to pass through the fruit opening during explosion without jamming as well as their ability to develop interesting mechanical properties due to the presence of the mucilaginous seed coat, when in contact with water are further important aspects of the E. elaterium dispersal mechanism that were in focus of this paper. To understand the structure-function relationship of the mucilaginous seed coat, we characterized the seed coat with scanning electron microscopy and performed adhesion characterization of the mucilage using micromechanical tests. Specifically, these insights yield a more detailed understanding of the elaborated seed dispersal mechanism of this plant that might serve as a basis for further upcoming bioinspired innovations.
Results
Internal morphology of the fruit
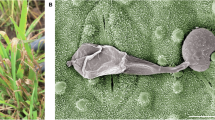
The fruits of E. elaterium grow on vertical stems over the leaves (Fig. 1a). The fruit wall consists of three different layers. The outer layer is the epidermis with its soft spines (Fig. 1b, c, i, j). Next to the epidermis is the resistance tissue, which contains the cells at the border to the inner parenchyma with the fruit mucus that generate the pressure in combination with the inner parenchyma finally causing the fruit explosion by an increase of the turgor. The center of the fruit consists of the inner parenchyma, in which the seeds are located on the median lines and the vascular bundles (Fig. 1b, d). The seeds are dark brown and have a light hilum (Fig. 1e), on which they are attached to the vascular bundles inside the fruit. After explosion, when seeds are hydrated and then dried in contact, they form a mucilaginous envelope (Fig. 1f) with a fiber array, which have a tree-like structure with loop ends (Fig. 1g, h).
Fruits on the stems in the natural environment (a). Longitudinal section of an exploded fruit (b–d) with the three different layers of the fruit wall (b, c). Three median lines in the middle of the fruit (b, d). Dry (e), hydrated (f) and dehydrated seed (g, h) with the fibers and the mucilaginous envelope, as the fibers were better visible after dehydration on a glass slide. A micro-CT scan based cross section of an intact (i) and exploded (j) fruit with the three layers of the wall and a seed attached to the vascular bundle. Ep epidermis, f fiber, ip inner parenchyma, me mucilaginous envelope, ml median line, rt resistance tissue, s seed, vb vascular bundle.
As it is difficult to open the ripened fruit of E. elaterium by cutting it, since it might explode and the arrangement of the seeds and other parts might change, the method of non-invasive µCT is a good approach to look inside the fruit. Here, we scanned a medium-ripe fruit and later generated a 3D reconstruction of the fruit and its inside. We were able to reconstruct the arrangement of the seeds at the vascular bundles, which formed a three-paired set of seeds arranged in six longitudinal rows (Fig. 2c). In the middle of the fruit, starting from the stem, three median lines of the vascular bundles run through the fruit. The fruit has in total 27 seeds, which are connected to the vascular bundles on the hilum and reached with their round ends to the median lines (Fig. 2c, d). Here, the number of seeds per longitudinal row varied (between 3 and 6 seeds per row). The stem itself sits on the fruit like a plug, which can simply be pushed out during the explosion for dissemination (Fig. 2a, b).
µCT based 3D images of a medium-ripe fruit. a View on the stem sitting in the base of the fruit and b hole in the head of the fruit after virtually removing the stem. The three-paired median line in the middle of the fruit is reaching to the hole and the stem and is connected to the resistance tissue. c Seeds are arranged in six longitudinal rows and are attached with the hilum to the three-paired vascular bundles. d The vascular bundles are partly connected to the median line. The seeds reach with their round end to the median line. Fr fruit, ml median line, s seed, st stem, vb vascular bundle.
Morphological changes of the plant stems with the fruit ripening
During ripening of the fruit, not only the fruit stem grows longer, but also the fruit itself changes its angle to the stem. Moreover, the stem straightens during ripening. We found that the riper the fruit, the higher the angle between fruit and stem (Fig. 3a, b). Ripe fruits showed a mean angle of 52.08 ± 8.96°. We found, all three angles are statistically significant different between different stages of the fruit ripening (One Way ANOVA, P < 0.001, F = 60.106; Tukey Test, all P < 0.001). While the angle is rising, the curvature of the stem is decreasing (Fig. 3c, d). The stems of unripe fruits showed a curvature of 0.02 ± 0.01 µm−1, of medium-ripe fruits 0.0095 ± 0.0034 µm−1 and of ripe fruits 0.0071 ± 0.0044 µm−1 with statistically differences between all three stages (Kruskal–Wallis One Way ANOVA on Ranks, P < 0.001, H = 49.374; Dunn’s Post-Hoc Test, all P < 0.05).
Angle between fruit and stem of unripe (N = 16), medium-ripe (N = 58) and ripe (N = 23) fruits (a) and during the ripening process (N = 5) (b). Curvature of the stem of c unripe (N = 33), medium-ripe (N = 83) and ripe fruits (N = 26) and d during the ripening process (N = 5). Different letters indicate statistically significant differences; a One Way ANOVA, P < 0.001; Tukey Test, all P < 0.001; c Kruskal–Wallis One Way ANOVA on Ranks, P < 0.001; Dunn’s Post-Hoc Test, all P < 0.05.
Dispersal of the seeds
We filmed the explosion of 7 fruits and calculated (1) the launching speed, (2) the speed after the seeds were ejected to the distance of 50 mm from the fruit and (3) the change of velocity with the time (acceleration). We found that there was no statistically significant difference in the launching speeds of the three seed categories (Kruskal–Wallis One Way ANOVA on Ranks, P = 0.663, H = 0.823). In contrast, middle seeds had statistically significant higher speeds after 50 mm than the first and last seeds (Kruskal–Wallis One Way ANOVA on Ranks, P < 0.001, H = 34.486; Dunn’s Post-Hoc Test, all P < 0.05) (Table 1). Interestingly, the last 2 ejected seeds were slower than all of the other seeds, but also had the smallest change of velocity with statistically significant differences between middle seeds and last 2 seeds (Kruskal–Wallis One Way ANOVA on Ranks, P < 0.001, H = 18.985; Dunn’s Post-Hoc Test, P < 0.05). We calculated the greatest explosion distance for the middle seeds and the smallest for the last 2 ejected seeds.
Structure of the mucilaginous coat of the seed and its mechanical properties
After contact with water, the seeds form a mucilaginous coat with fibers within the coat. In addition, the fruit emits a mucus while exploding and dispersing the seeds. We imaged the seeds and associated fluids in different stages, 1) the dry, intact seed (Fig. 4a, b), 2) the mucus ejected by the fruit (Fig. 4c), 3) the seed soaked in the fruit mucus (Fig. 4d–g) and 4) the seeds after hydration and subsequent drying (Fig. 4h–j). We found that the seeds soaked in the fruit mucus caused spreading of a minor amount of fibers from the seed coat (Fig. 4e). Moreover, the seed coat seems to be swollen and shows several openings and possible terpene crystals (Fig. 4f, g). After hydration, the fibers, which were spread due to the seed contact with water, were anchored to the seed coat and adhered to the glass plate, when the seed was drying in contact (Fig. 4i, j). Surprisingly, staining for cellulose on hydrated seeds did not show any reaction. The fibers seem to have several segments, which are assembled into very long and folded sections (Fig. 4i). The fibers have an approximate diameter of 1–2 µm (Fig. 4j). Staining for pectins did show a positive reaction and confirmed the presence of pectins within the mucilage (see Supplementary Fig. 3).
Dry intact seed (a, b). Residues of the fruit mucus, dried in contact with a glass plate (c). The seed soaked in the fruit mucus and afterwards dried (d–g). Hydrated and afterwards dried seed (h–j). Arrows show spread fibers on the seed coat (e). Numerous openings and terpene crystals are seen on the seed coat (f, g). Numerous spread and dried fibers on the seed coat after hydration. Fibers seem to have several segments/sections (i). F fiber, op openings, sc seed coat, tc terpene crystal.
To test, whether the fruit mucus might reduce friction of the seeds while leaving the fruit at the beginning of explosion, we performed friction tests. Here we found that the friction force of the seeds soaked in the fruit mucus slightly varied from 3.5 to 10.2 mN over the time period of approx. 15 min between measuring the first and last seeds, with a mean and standard deviation of 6.66 ± 1.96 mN (Fig. 5a). The linear regression, however, showed a weak linear dependence (R2 = 0.216) and no statistically significant dependence of friction force on time (Analysis of Variance, P = 0.176, F = 2.199). Friction coefficients varied strongly from 0.09 to 0.34 with a mean and standard deviation of 0.218 ± 0.080 (Fig. 5b), there was no statistically significant dependence of friction coefficient on time (Analysis of Variance, P = 0.922, F = 0.0103; with R2 = 0.0013). The dry seeds had a mean friction force of 2.23 ± 1.49 mN and a mean friction coefficient of 0.260 ± 0.055. The mean friction forces showed statistically significant differences between dry seeds and seeds soaked in fruit mucus (One-tailed t-test, P < 0.0001, t = 5.435). The mean friction coefficients of dry seeds were significantly higher than those of seeds soaked in fruit mucus (One-tailed t-test, P = 0.0304, t = −2.0).
Friction force (a) and friction coefficient (b) of the seeds soaked in the fruit mucus and dry seeds (experiments performed with the tilting platform). The linear regression of the friction force with weak linear dependence (R2 = 0.216), but no statistically significant dependence (Analysis of Variance, P = 0.176). The fruit mucus was fresh at beginning of the first test run. Friction tests were performed within 30 min. Adhesion force (N = 17) (c) and adhesion strength (N = 17) (d) of dried seeds previously soaked in the fruit mucus (experiments performed with the Biopac testing device) and seeds with dried seed mucilage (experiments performed with the Zwick testing device). Different letters indicate statistically significant differences. c, d Mann–Whitney Rank Sum Test, both P < 0.001.
Seeds which were soaked in the fruit mucus and air dried in contact with glass showed only small adhesion forces with a mean and standard deviation of 0.23 ± 0.25 N and adhesion strength of 40 ± 40 KPa. Seeds, which were hydrated and formed the mucilage coat around the seeds and subsequently air dried on glass, showed a mean adhesive force of 12.4 ± 7.9 N and an adhesive strength of 480 ± 330 KPa. Both, the adhesion force (Fig. 5c) and the adhesive strength (Fig. 5d) showed statistically significant differences between seeds soaked in the fruit mucus and seeds with surrounded with own seed coat mucilage (Mann–Whitney Rank Sum Test, adhesion force: P < 0.001, T = 153.00, U Statistic = 0.0, adhesive strength: P < 0.001, T = 157.0, U Statistic = 4.0).
Discussion
We were able to reconstruct the internal morphology of the fruit of E. elaterium with the seeds arranged in six longitudinal rows, as it was already previously schematically described and supported by recent µCT results12,17. The seeds, which are attached to vascular bundles, are all connected by these bundles to three median lines in the middle of the fruit (Fig. 2c, d). Sadly, we were not able to fully reconstruct the median lines and the vascular bundles due to limitation of the µCT scan resolution, but the median lines apparently stay at their place even after the explosion and dispersal of the seeds (Fig. 1b, g). We were able to record, that each seed had left the fruit with the rounded pole first (Supplementary Movie 1). Here, the median lines might aid in stabilization and holding the seeds ‘in line’ for a controlled dispersal direction and seed orientation during their flight phase, while the longitudinal rows are important for the pre-orientation of the seeds, which are suspended with the rounded ends inside the fruit. With the exploding parenchyma cells, the mucus of the fruit is poured out17 which guides the seeds from the middle of the fruit to the outside through the opening. Therefore, the seeds might turn their rounded ends towards the opening within the mucus stream and leave the fruit in a row starting with the seeds closest to the stem, ending with the seeds at the other end of the fruit. Previous studies suggested, that the mucus (or ‘cell sap’) is secreted from the swelling tissue (inner parenchyma)11. Using CT, we corroborate that the cells of the resistance tissue were emptied and enlarged after the explosion, which confirms that the fruit’s mucus is produced in the cells of the resistance tissue at the border to the inner parenchyma.
As the fruits ripen, not only the stems of the fruits get straightened, but also the angle between fruit and stem changes, to reach an angle best suitable for the seed dispersal. It is already stated, that the perfect theoretical angle for the ballistic parabola is about 45° without considering air resistance and approximately 50° under consideration of air resistance7,24. Still, both angles are not directly reached in this study. The angle of the fruits is about 55°, and the shooting angle about 53–55° 7,15. We were able to confirm the previously measured angles of ripe fruits15, but our measured launching angle differs with the launching angle of 42.7 ± 8.9° measured by Box et al. (2024), which comes closer to the perfect theoretical angle without air resistance12. This angle might help the plant to disperse the seeds with greatest distance from the plant, but also makes sure that the seeds are ejected over the adjacent leaves of their own and neighboring plants7.
With the help of high-speed videography, we were able to calculate the speeds of a reasonably high number of seeds. The first seeds were somewhat slower than the following seeds and the last seeds were the slowest. Moreover, the middle seeds reached significantly higher speeds after some initial distance in the range of few millimeters, with a statistically significant higher change of velocity. It was stated that the first ejected seeds were fastest then the speed decreased continuously8,14. We were not able to confirm this statement. However, differing ejection velocities might result from the seeds arrangement in the fruit. As we stated before, the first seeds which leave the fruit might be the ones closest to the stem/fruit opening and therefore cannot achieve a high launching speed since they are accelerated by the squeezing force form the pericarp for a shorter distance. In contrast, the last ejected seeds, which might be arranged at the other end of the fruit, cannot obtain higher kinetic energy due to the small remaining elastic potential energy of the pericarp. The middle seeds, which reached the highest speeds measured in this study can have a sufficient acceleration distance resulting in enough energy from the pericarp. Additionally, with the varying speeds of seeds during the explosion, we calculated different explosion distances for these three groups of seeds. Ranging from 9.71 to 12.04 m, the seeds might reach rather large distances for the distribution of offspring plants. Since the stems of the fruits are not unambiguously straight, the seed dispersal distances might slightly vary due to the decreasing turgor during the explosion, but Overbeck (1930) directly measured dispersal distances of up to 12.7 m7, which perfectly correspond to our estimations based on the high-speed video analysis and the data from other studies12. Further validation of this might need more experiments and physical model constructions to find out more about the order of seeds leaving the fruit and the initial arrangement within the fruit and relationship between this seed arrangement and flight direction.
The seeds of E. elaterium show a special structure when hydrated. Here, the seed forms a mucilage with a fiber skeleton around the seed coat16,21. This mucilage resembles the mucilage of several other plant seeds, e.g. Plantago ovata, Salvia hispanica, and Arabidopsis thaliana with cellulose fibers as a kind of skeleton for the mucilage25,26,27. The seeds of E. elaterium also showed fibers, but staining for cellulose did not show any reaction, which means that these fibers are not composed of cellulose as previously suggested21. In seeds of other plants, the mucilage together with its fibers is activated by the contact with water. Here it seems that some individual fibers are already spread from the seed coat in contact with the fruit mucus, which mainly consists of an aqueous solution of elaterin and Cucurbitacin E with a pH of 5.617,28. Other studies on flax seeds already showed that mucilage extraction with a neutral medium, such as water, showed better quality than in acidic medium, but still, more acidic media did not inhibit the formation and extraction of the seed mucilage29. Most of the fibers and mucilage still develop from the seed coat after the seed contact with water. Nevertheless, the fruit mucus influences the seed coat in other ways. Holes and their surroundings (Fig. 4e–g) might come from precipitated salts of the elaterin, which then cause holes on the surface of the bloating seed coat. In addition, many terpene crystals, which are clumped together, are visible on the seed surface next to the holes. These terpene (or Cucurbitacin E) crystals have strong similarities with those of Cucurbitacin B30,31.
In general, the fruit mucus might slightly help the seeds to smoothly exit the fruit and already start to slightly adhere to surfaces. With a friction coefficient (µ) of 0.218 ± 0.080 of in the fruit mucus–soaked seeds, their frictional properties are slightly smaller than of dry seeds, e.g. of Linum usitatissimum with µ of 0.25–0.3932. Still, µ of in the fruit mucus–soaked seeds is still higher than the one of hydrated seeds of L. usitatissimum (µ = 0.039–0.055)32. Dry seeds of E. elaterium (µ = 0.26 ± 0.055) showed similar friction coefficients as the dry seeds of L. usitatissimum. However, E. elaterium seeds soaked in the fruit mucus did not show high adhesive forces (0.23 ± 0.25 N). Regarding the relatively high friction forces and the low adhesive forces, the fruit mucus might only be important for the explosion initiation of the fruit by the release of the mucus from the cells of the resistance tissue17.
In contrast, seeds which were hydrated with water and later dehydrated on glass, showed very high adhesive forces of up to 27.5 N and a mean of 12.4 ± 7.9 N. Earlier studies on the adhesive properties of E. elaterium seeds suggested forces of about 535 pound, which is about 5.25 N9. Other seeds with and without cellulose fibers showed smaller adhesive forces than the seeds of E. elaterium. Pectic seeds, such as L. usitatissimum, showed maximum adhesion forces of 2.1 N, whereas cellulosic seeds, e.g. Ocimum basilicum or Salvia hispanica showed maximum adhesion forces of 6.22 N and 3.24 N. The hemicellulosic seeds, such as Plantago ovata, showed maximum adhesion forces of up to 5.74 N33. The adhesion forces of the seeds of E. elaterium surpass all other ever measured adhesion forces of myxospermous seeds; however, the adhesive strength of 0.48 ± 0.33 MPa is rather comparable to other seeds. Pectic seeds of L. usitatissimum had a mean adhesive strength of 1.41 ± 0.86 MPa, cellulosic ones of O. basilicum of 1.55 ± 0.55 MPa and S. hispanica of 1.00 ± 2.65 MPa and hemicellulosic ones of P. ovata of 0.94 ± 1.89 MPa. Similar to other seeds, such as Coccinia grandis, Ecballium elaterium seeds have a larger contact area than other seeds and therefore have clearly smaller adhesive strengths34.
This study gives new insights in the properties of the dispersal mechanism of the squirting cucumber E. elaterium. and further confirms findings of other recent studies12. With new methods, we were able to reconstruct the inside of the fruit in 3D, morphological changes of plant stems during the fruit ripening process and details of the seed dispersal during the explosion process. Shortly before the explosion, the fruit reaches its optimal arrangement for the most effective ballistic characteristics. Furthermore, we found that the seeds have strong adhesive properties, when hydrated and dried in contact, which might be an adaptation to improved seed contact with the soil, seed protection from the drought and enhanced germination conditions, which are important requirements for the development of new plants. We can conclude, that with newer experimental techniques (such as µCT and 3D reconstruction), we can get a better insight in the fruits inner structure without damaging the fruits. This can help in future simulations, with the addition of the here observed and measured morphological changes and adaptations of the fruit. With the example of E. elaterium conclusions can be drawn about similarly functioning seed dispersal mechanisms and can further aid in creating robotical systems for industrial applications.
Methods
Plants and fruits
Fruits of Ecballium elaterium were collected in Alaçatı near Çeşme/İzmir (Turkey, 38°19'35.8“N 26°20'26.2“E) and Perugia (Italy, 43°06'06.7“N 12°23'22.2“E) to perform microcomputed tomography (µCT), scanning electron microscopy (SEM) and high-speed video (HSV) recordings. Photos of 142 fruits were taken in Alaçatı (Turkey) in their natural environment, to later analyze stem curvature and fruit-stem angle at different stages of ripening. Furthermore, plants from the Botanical Garden Kiel (Germany, 54°20'51.4“N 10°07'01.7“E) were observed, photographed and used for HSV recordings and studies of the stem curvature and fruit-stem angle during the ripening process of the fruits. Dark green fruits were considered as unripe, lighter greenish/yellowish fruits were considered as medium-ripe and yellow fruits were considered as ripe (see Supplementary Fig. 1). To investigate how the angle between fruit and stem changes before reaching the final angle at which seeds are catapulted, we analyzed the angles of 97 individual fruits and additionally measured the stem curvature of 142 plants from 126 images taken in the natural habitat of E. elaterium. Furthermore, we analyzed the angle between fruit and stem and curvature of the stem of 5 plants, of which we took a photo every day during the ripening process.
Microcomputed tomography
Medium-ripe fruits were placed in plastic tubes for µCT scans. The scans were carried out using a Skyscan 1172 micro-CT (Bruker micro-CT, Kontich, Belgium; CT-scanner settings: X-ray source: 100 kV, 100 µA, aluminum filter, 360° rotation, 0.2 rotation step, 10 frame averaging and 10 random movement; image pixel size: 13.38 µm, reconstructed in Nrecon 1.0.7.4 (Bruker micro-CT, Kontich, Belgium), processed with Amira 6.2 (Thermo Fisher Scientific, Waltham, USA) and subsequently visualized using the open-source 3D creation suite Blender 2.82a (Blender Foundation, Amsterdam, the Netherlands).
High-speed video recordings
We recorded the explosion of the fruit with a Sony RX10III camera (Sony corporation, Tokyo, Japan) at 1,000 frames per second at the growing site by placing the very ripe fruit in focus of the camera and touching the fruit with a stick to trigger the explosion. Moreover, we recorded a forced explosion of a medium-ripe fruit with a Photron Fastcam SA1 high-speed camera (Photron Europe Ltd., West Wycombe, Bucks, UK) at 10,000 frames per second under laboratory conditions by pressing the fruit until the stem detached and seeds were thrown out.
To analyze the HSV recordings, we used the image software ImageJ (ImageJ2, V: 2.14.0/1.54f)35. Each video recording was then analyzed frame by frame, the distances of the seeds were measured and the speed and possible shooting distance of the seeds were calculated.
We filmed the explosion of 7 fruits and calculated (1) the launching speed, (2) the speed after the seeds were ejected to the distance of 50 mm from the fruit and (3) the change of velocity with the time (acceleration = (speed-launching speed)/time). For images of the seeds at the launching stage and after 50 mm (see Supplementary Fig. 2). Here we categorized the seeds in the first 2 ejected seeds, the following middle seeds (varying numbers of seeds per fruit) and the last 2 ejected seeds. With the previously measured angle between the fruit and stem (52.08° ± 8.96°) of ripe fruits, we were able to estimate the mean throwing angle with following equation:
with θ, as the throwing angle, and α, as the measured angle between the fruit and stem. With these values, we were able to estimate the possible explosion distance of these three seed categories. As the drag of the seeds was very low (2.34 × 10−6 m−1), we were able to disregard the drag when calculating the explosion distance. The fruits grow with a mean height of 17.5 cm above the ground7. Since the curvature of the stem of ripe fruits was very small, we disregarded the curvature and have assumed a straight stem. Therefore, we used following equation to estimate the possible explosion distance (L) of the seeds:
With v0 = launching speed, g = gravitational acceleration and h = fruit height.
Scanning electron microscopy
For scanning electron microscopy (SEM), the seeds of exploded fruits were either dry, soaked in the fruit’s mucus or air-dried on a flat surface. For the latter, the seeds were hydrated for 30 min in distilled water and then individually transferred to a glass surface. After drying, all seeds were mounted on metal stubs, sputter-coated with a 10 nm layer of gold-palladium (Leica Bal-TEC SCD500, Leica, Wetzlar, Germany) and visualized with the scanning electron microscopes SEM Hitachi S-4800 (Hitachi High-technologies Corp., Tokyo, Japan) and Hitachi TM3000 (Hitachi High-technologies Corp., Tokyo, Japan) at accelerating voltages of 3 kV and 15 kV, respectively.
Friction and adhesion measurements of the seeds
For the friction measurements, a custom made tilting platform32,36 was used. Seeds were tested in two different conditions: seeds obtained after fruit explosion without further treatment (dry seeds) and seeds additionally soaked for up to 15 min in the fruit’s mucus after fruit explosion. The seeds, which were soaked in the mucus, were all collected at once and then tested one after another. Therefore, the seeds were stored in the mucus in a plastic bad to avoid contact with air, until used for friction tests. We used 10 groups of 3 seeds each with 4 sets of repetitions with different normal load for friction measurements for each seed condition. Dry seeds and seeds soaked in the fruit’s mucus were freshly prepared after the fruit explosion and placed on a flat horizontal platform, capable of rotating at different speeds. A glass block of a certain mass was then placed on a group of three seeds. To test the friction coefficient between the seeds and glass at different load forces, we used four glass blocks for applying different loads: (1) 19.7 mN, (2) 36.4 mN, (3) 56.8 mN and (4) 74.5 mN. Beginning with the lowest load and ending with the highest, the experiment was repeated with varying load. For the measurement the platform was tilted at slow speed (5°/sec). As soon as the glass block started sliding, the tilting was stopped, and the tilt angle was determined. After every measurement, the glass block was cleaned to prevent the influence of remains of the mucus. The friction force (Ffr,ad) in the presence of adhesion was determined using following equation:
Where µ is the friction coefficient and Fn and Ffr are normal and friction force, respectively. µ and Ffr,ad were obtained from (2) following Kreitschitz et al.32. Values of Ffr,ad ≤ 0 were omitted from the dataset.
Pull-off force measurements were performed to detach the dried-in-contact seeds from a glass plate. To compare the adhesive properties of the seed envelope between in water and mucus-soaked seeds, the seeds were either hydrated in distilled water or soaked in the fruit mucus. Then the seeds were placed on glass plates and let dried in contact. In total, 17 water-hydrated seeds and 17 seeds soaked in the mucus were tested. After drying, the contact area of the mucilage to the glass was measured with a 3D measurement microscope (Keyence VR 3100, KEYENCE, Neu-Isenburg, Germany). For the force measurements of the seeds soaked in mucus, a BIOPAC MP 100 data acquisition system (BIOPAC System Inc, Goleta, USA) was equipped with a Fort1000 force transducer (1000 g capacity, World Precision Instruments Inc., Sarasota, USA). The force transducer was fixed to a motorized micromanipulator DC3314R with a controller MS 314 (World Precision Instruments, Sarasota, USA). The glass plate with the seeds adhering to it was mounted on another glass plate with double-sided tape. Then, a screw, mounted on the force transducer, was glued to the seed with UV glue (BONDIC GmbH, Köln, Germany). Here, only the seed, but not the mucilage on the glass plate was in contact with the glue. Tension was applied by moving the force transducer down and thereby pulling the seed off the glass. By using the software AcqKnowledge 3.7.0 (BIOPAC System Inc, Goleta, USA), force-time curves were recorded and the maximum adhesion forces Fad were determined.
For the measurements of the dried-in-contact seeds with the hydrated mucilage envelope, the material tester Zwick Roell z 0.5 (Zwick GmbH & Co. KG, Ulm, Germany, Type: BT1-FR0.5TN.D14) was used. Here, the glass plate with the attached seeds was mounted with double-sided tape on a platform on the force transducer. The seeds were then glued with epoxy (R&G Faserverbundwerkstoffe, Waldenbuch, Germany) on a screw already mounted to the force sensor. After the epoxy glue has dried out (after approx. 30), the force transducer was pulled upwards to pull the glass off the seeds with the dried mucilage envelope. The force-distance curves were continuously recorded during the pulling and the maximum adhesion force Fad was recorded. Using this force values and the contact area A0 of the mucilage to the glass, the adhesion strength σc was determined with
To test for cellulose and pectin in the hydrated seeds, seeds were stained with Calcofluor White (0.25% w/v in distilled water) for cellulose and Ruthenium Red (0.05% w/v in distilled water) for pectins (see Supplementary Fig. 3). Therefore, the seeds were extracted from an intact fruit without triggering the explosion and were then hydrated in distilled water for 30 min. Then, they were stained with the staining for 5 min with Calcofluor White or 1 min with Ruthenium Red, respectively, and observed with a microscope (Zeiss Axioplan, Carl Zeiss Microscopy GmbH, Jena, Germany).
Data analysis
Measurements of the fruit-stem angle were performed with the angle tool of the image analysis software Fiji37. Here, a straight line was laid through the length of the fruit (with its center at the base of the stem on the fruit) and the second straight line through the stalk (for detail, see Supplementary Fig. 4). For the measurements of the stem curvature, we used the curvature plug-in Kappa38 (available at https://imagej.net/plugins/kappa) for Fiji and measured the curvature of the entire visible stem from the highest point to lowest point of stem (Supplementary Fig. 4). We measured the angle and curvature three times for each fruit and continued our analysis with the mean of the three measurements.
Statistical analyses were performed with SigmaPlot software for Windows (12.5; Systat Software, San Jose, CA, USA). All datasets were tested for normality (Shapiro–Wilk) and homogeneity of variance (Levene) and according to the results of these tests, either parametric or non-parametric tests were used for further statistical analyses.
Data availability
The data that supports these findings are openly available in https://doi.org/10.6084/m9.figshare.26268262.
References
Howe, H. F. & Smallwood, J. Ecology of Seed Dispersal. Annu. Rev. Ecol. Syst. 13, 201–228 (1982).
Murray, D. R. Seed Dispersal (Elsevier Science, 2014).
Schupp, E. W., Jordano, P. & Gómez, J. M. Seed dispersal effectiveness revisited: a conceptual review. New Phytol. 188, 333–353 (2010).
Sakes, A. et al. Shooting Mechanisms in Nature: A Systematic Review. PLOS ONE 11, e0158277 (2016).
Vittoz, P. & Engler, R. Seed dispersal distances: a typology based on dispersal modes and plant traits. Bot. Helvetica 117, 109–124 (2007).
Fahn, A. Nectary Structure and Ultrastructure of Unisexual Flowers of Ecballium elaterium(L.) A. Rich. (Cucurbitaceae) and their Presumptive Pollinators. Ann. Bot. 87, 27–33 (2001).
Overbeck, F. Mit welchen Druckkräften arbeitet der Schleudermechanismus der Spritzgurke? Untersuchungen an Ecballium elaterium Rich. Planta 10, 138–169 (1930).
Wolters, B. Hochfrequenzkinematographische Untersuchung des Turgor-Spritzmechanismus von Ecballium. Planta 60, 344–348 (1963).
Grubert, M. Studies on the distribution of myxospermy among seeds and fruits of Angiospermae and its ecological importance. Acta Biol. Venez. 8, 315–551 (1974).
Guttenberg, H., Tischler, G. & Linsbauer, K. Handbuch Der Pflanzenanatomie (Borntraeger, 1971).
Roth, I., Linsbauer, K., Tischler, G. & Pascher, A. Handbuch Der Pflanzenanatomie (Gebrüder Borntraeger, 1977).
Box, F. et al. Uncovering the mechanical secrets of the squirting cucumber. Proc. Natl. Acad. Sci. 121, e2410420121 (2024).
Hildebrand, F. Die Schleuderfrüchte und ihr im anatomischen Bau begründeter Mechanismus. In: Jahrbuch f. wiss. Botanik vol. 9 235–276 (Pringsheim, 1873).
Obaton, M. F. Sur la projection des graines de l’ Ecballium Elaterium Rich. Bull. Société Bot. Fr. 94, 95–98 (1947).
Straka, H. Nicht durch Reize ausgelöste Bewegungen. In: Physiology of Movements/Physiologie der Bewegungen, 716–835 (Springer, 1962). https://doi.org/10.1007/978-3-642-94852-7_19.
Braemer, L. De la localisation des principes actifs des curcubitacées: Recherches histologiques et histochimiques. In: Bulletin de la Société d’histoire naturelle de Toulouse (Lagarde et Sebille, 1893).
Guttenberg, V. H. Zur Kenntnis des Spritzmechanismus von Ecballium Elaterium Rich. Berichte der Deutschen Botanischen Gesellschaft 33, 20–37 (1915).
Roze, M. E. Le fruit de l’Ecballium elaterium Rich. (Momordica elaterium L.). J. Bot. 8, 308–318 (1894).
Casper, B. B., Heard, S. B. & Apanius, V. Ecological correlates of single-seededness in a woody tropical flora. Oecologia 90, 212–217 (1992).
Kayahan, N. Assessment of Some Physical and Mechanical Properties of Ecballium Elaterium (L.) Seeds Grown in Natural Environment. Int. J. Life Sci. Agric. Res 3, 490–496 (2024).
Fickel, J. F. Ueber die Anatomie und Entwicklungsgeschichte der Samenschalen einiger Cucurbitaceen. Bot. Ztg. Bd. 34, 738–778 (1876).
Lewes, D. Observations on the Internal Pressure of the Ripening Fruit of Ecballium Elaterium. Kew Bull. 6, 443 (1951).
Wang, X. et al. Fracture-driven power amplification in a hydrogel launcher. Nat. Mater. https://doi.org/10.1038/s41563-024-01955-4 (2024).
Price, R. H. & Romano, J. D. Aim high and go far—Optimal projectile launch angles greater than 45°. Am. J. Phys. 66, 109–113 (1998).
Western, T. L. The sticky tale of seed coat mucilages: production, genetics, and role in seed germination and dispersal. Seed Sci. Res. 22, 1–25 (2012).
Kreitschitz, A., Kovalev, A. & Gorb, S. N. Sticky invasion” – the physical properties of Plantago lanceolata L. seed mucilage. Beilstein J. Nanotechnol. 7, 1918–1927 (2016).
Kreitschitz, A. & Gorb, S. N. How does the cell wall ‘stick’ in the mucilage? A detailed microstructural analysis of the seed coat mucilaginous cell wall. Flora 229, 9–22 (2017).
Greige-Gerges, H. et al. Cucurbitacins from Ecballium elaterium juice increase the binding of bilirubin and ibuprofen to albumin in human plasma. Chem. Biol. Interact. 169, 53–62 (2007).
Rocha, M. S., Rocha, L. C., Feijó, M. B. D. S., Marotta, P. L. L. D. S. & Mourao, S. C. Effect of pH on the flaxseed (Linum usitatissimum L. seed) mucilage extraction process. Acta Sci. Technol. 43, e50457 (2021).
Cheng, L. et al. Improve bile duct-targeted drug delivery and therapeutic efficacy for cholangiocarcinoma by cucurbitacin B loaded phospholipid complex modified with berberine hydrochloride. Int. J. Pharm. 489, 148–157 (2015).
Wu, Q. et al. Preparation, characterization and pharmacokinetics of Cucurbitacin B solid dispersion. OpenNano 8, 100088 (2022).
Kreitschitz, A., Kovalev, A. & Gorb, S. N. Slipping vs sticking: Water-dependent adhesive and frictional properties of Linum usitatissimum L. seed mucilaginous envelope and its biological significance. Acta Biomater. 17, 152–159 (2015).
Kreitschitz, A., Kovalev, A. & Gorb, S. N. Plant Seed Mucilage as a Glue: Adhesive Properties of Hydrated and Dried-in-Contact Seed Mucilage of Five Plant Species. Int. J. Mol. Sci. 22, 1443 (2021).
Büscher, T. H. & Gorb, S. N. Convergent Evolution of Adhesive Properties in Leaf Insect Eggs and Plant Seeds: Cross-Kingdom Bioinspiration. Biomimetics 7, 173 (2022).
Schneider, C. A., Rasband, W. S. & Eliceiri, K. W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675 (2012).
Berthé, R. A., Westhoff, G., Bleckmann, H. & Gorb, S. N. Surface structure and frictional properties of the skin of the Amazon tree boa Corallus hortulanus (Squamata, Boidae). J. Comp. Physiol. A 195, 311–318 (2009).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Mary, H. & Brouhard, G. J. Kappa (κ): Analysis of Curvature in Biological Image Data Using B-Splines. http://biorxiv.org/lookup/doi/10.1101/852772 (2019).
Acknowledgements
We would like to thank Susanne Petersen, Oliver Klemme and their team (Botanical Garden at Kiel University, Germany) and Valerio Saitta and Manuela Rebora (University of Perugia, Italy) for the provision of the plants. Furthermore, we thank Lotte Blankenburg, Esther Appel, Alexander Kovalev, Mohsen Jafarpour and Chuchu Li for technical support during the experiments and Anika Preuss, Fabian Bäumler, Julian Thomas, Benedikt Josten and Simon Züger for advice and support during this study. Funding to S.N.G. by the grant GO 995/43-1 from German Science Foundation (DFG) is greatly acknowledged. The funders took no part in the study design, data collection and analysis, decision of publishing or any preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
H.G., S.N.G. – Conceptualization; H.G., M.B., P.S. – Data curation; H.G., M.B., T.H.B. – Data analysis; H.G., S.G. – Methodology; H.G., T.H.B. – Investigation; H.G. – Visualization; H.G. – Writing – original draft; M.B., P.S., T.H.B., S.N.G. – Writing – review & editing; S.N.G. – Funding acquisition.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gorges, H., Brinkhaus, M., Sator, P. et al. Structural and biomechanical adaptations of fruits and seeds in Ecballium elaterium (Cucurbitaceae) for seed dispersal. npj Sci. Plants 1, 4 (2025). https://doi.org/10.1038/s44383-025-00003-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s44383-025-00003-7