Abstract
Tetragonal (t)-LaVO4 has turned out to be a potential host for luminescent materials. Synthesis of t-LaVO4 till date has been based on chelating effect of EDTA making it not ideal for bioimaging applications. An alternative was proposed by us through the use of catechin. In recent times there is interest for new MRI contrast agents that can through appropriate doping function both as MRI contrast and optical/upconversion materials. It is generally believed that under appropriate doping, t-LaVO4 would be a better upconversion material than monoclinic (m)-LaVO4. Based on these postulations, this work explores the use of gadolinium doped t-LaVO4 as an MRI contrast agent. From literature, gadolinium oxide is a good T1 contrast agent. Through this work, using catechin as a template for the synthesis of Gd doped t-LaVO4, we demonstrate the possible use as a T1 contrast agent. Interestingly, as the catechin concentration changes, morphology changes from nanorods to square nanoplates and spheres. In this process, a switch from T1 to T2 contrast agent was also observed. Under optimal concentration of catechin, with a rod shaped Gd doped t-LaVO4 an r2/r1 value of 21.30 was observed. Similarly, with a spherical shape had an r2/r1 value of 1.48 was observed.
Similar content being viewed by others
Introduction
In recent years, efforts to couple imaging modalities such as optical and magnetic resonance imaging have met with success. Such coupling brings to the fore the advantages of both the methods, say, with respect to imaging resolution and penetration depth1,2. Magnetic resonance imaging (MRI) is a non-invasive method to diagnose diseases owing to its high spatial resolution and good soft-tissue contrast. It works either by shortening the longitudinal (T1) or the transverse (T2) relaxation time of water protons. MRI contrast agents (CAs) can be classified as either positive (T1) CAs or negative (T2) CAs. Typically, MRI signal obtained by T2 CAs is easily confused with other artifact signals, like calcification, bleeding, and metal deposits, Etc. Generally positive T1 CAs is widely used as extracellular, hepatobiliary, and blood pool agents in medical imaging. Positive T1 CAs gained its advantage for a bright MR image, high longitudinal relaxation rate, low cytotoxicity, and low intake dose. Currently, Gadolinium chelates like Gd-DOTA and Gd-DTPA are used as T1 CAs but release a certain amount of free Gd ions, which inhibit calcium channels which leads to cardiovascular and neurologic toxicity. Also, Gd-based inorganic nanoparticles, such as carbonate (Gd2 (CO3)3), fluoride (GdF3, NaGdF4), oxide (Gd2O3), and vanadate (GdEuVO4) were investigated3. The advantage of doping Gd ions into the host crystal structure lies in a very low leaching of free Gd ions and even more stable than Gd based MRI contrast agents4. In Gd doped host lattices, surface Gd3+ ions offer all seven of its unpaired electron for water hydration by Inner sphere contribution, which cooperatively induce the longitudinal relaxation of water proton. Gd3+ chelates can only offer one hydrate position since their other six unpaired electrons are coordinated by chelates. This synergistic effect enhances relaxivity value of Gd doped host lattices than Gd chelates3. Nanostructures based on Gd3+ doped NaYF4, codoped with Yb3+/Er3+ has been reported for upconversion imaging coupled with MRI5. Unfortunately, fluorides tend to be hygroscopic and have less favorable chemical and photophysical stabilities6.
Lanthanide orthovanadates are potential hosts for luminescent materials, when in appropriate matrices. LaVO4 exists in two phases, viz., the monoclinic, monazite structure and tetragonal zircon structure. La3+ generally prefers the monazite structure as the thermodynamically stable state. While m-LaVO4 is not a suitable host for luminescent activators, t-LaVO4 is a promising phosphor. This variability in the properties of the polymorphs, had created an extensive interest in selective synthesis and phase change processes7. Through the years, hydrothermal method based on EDTA has emerged as an effective way to synthesize the metastable t-LaVO48.
Though t-LaVO4 has emerged as a potential luminescent material, the success of the same as an upconversion phosphor has been limited. It is only recently that Singh et al.9 and Zheng et al.10 reported the upconversion properties of Yb3+/Er3+ doped t-LaVO4. No such reports were found in the literature for Gd-doped t-LaVO4. One of the challenges for the bioimaging applications of the orthovanadate is in the use of EDTA for the selective synthesis of t-LaVO4. EDTA is known to bring about cytotoxicity11.
Encouraged by the need to overcome the drawbacks, we for the first time report catechin assisted t- Gd-doped lanthanum vanadate (GL) nanoparticles with varying morphologies as T1 contrast agents. Catechin possesses a large number of phenolic hydroxyl groups susceptible for metal chelation, and its biocompatibility led to the choice compared to that of other existing additive systems like EDTA, and citric acid. As structure of catechin is pH dependent, it is stable in highly acidic solution and unstable in neutral or alkaline solution. In general, Catechin is absorbed from the human intestinal tract, largely metabolized and distributed as conjugated derivatives in blood, and that these forms are excreted in urine12. Catechin is known for its superior hydrophilic antioxidant property because of its higher number of hydroxyl groups and retards lipid oxidation. It can scavenge hydroxyl, peroxyl, and 2,2-diphenyl-1-picrylhydrazyl (DPPH) radicals. The thermal stability of catechin in the presence of oxygen is 227 °C and weight loss from 50 to 110 °C is due to water evaporation13. Catechin interacts with plasma proteins through different covalent and noncovalent bonds (i.e., hydrogen bonding, π-bonding, hydrophobic, and ionic interactions), and brings about significant changes in structure, physicochemical properties, and the activity of proteins14. It has a strong affinity with lipid bilayers, which facilitates their entry into cancer cells15. Since catechin is known for its beneficial effects like antioxidative, anticancer, anti-inflammatory and antithrombogenic activities7,11,16 it is expected that catechin modulated hydrothermal synthesis could offer a 3-fold advantage, viz., phase, morphology, and magnetization directed synthesis.
Results and Discussion
The role of catechin hydrate (cat) in polymorph selection for a doped system can be seen from Fig. 1. The product obtained from the hydrothermal treatment of La(NO3)3.6H2O, Gd(NO3)3.6H2O and Na3VO4 in the absence of catechin, was well indexed to m-LaVO4 (JCPDS No. 500367) with a space group (p21/n)], cell parameters a = 7.043 Å; b = 7.279 Å; c = 6.721 Å and cell volume = 333.071 Å3 (Fig. 1A(a)). Incorporation of cat as ligand accelerates the formation of tetragonal phase with cell parameters a = b = 7.4578 Å; c = 6.5417 Å and cell volume = 363.841 Å3 [JCPDS no. 10–705226; space group I41/amd (141)] as shown in Fig. 1A(b). It can thus be seen that at an appropriate concentration of cat, the formation of pure t-LaVO4 without the presence of impurity phases such as m-LaVO4 is possible, similar to our earlier observations (Fig. 1B). Catechin act as the capping as well as stabilizing agent by the interaction of Ln3+ (Ln = La, Gd) with phenolic OH groups at 5, 7, 3′, 4′ positions (Fig. 2)17. Well-resolved peaks, as can be seen in Fig. 1B, indicates a highly crystalline nature, alongside lower defects, the added advantage being the use of low hydrothermal treatment temperature (180 °C). Sharp peaks with even peak profiles coupled with highly crystalline nature is an indication of smaller crystallite sizes. This can be further confirmed from the crystallite size calculated by Debye-Scherer formula. Cell parameters thus obtained are provided in Table 1. Lattice strain calculated by Williamson-Hall (W-H) method employing the plot in Fig. 1C, further demonstrates the formation of the metastable state. Crystal structure of five GL nanoparticles was established from Rietveld structural refinement of slow scan powder XRD data (Fig. 3).
XRD pattern of GL nanoparticles (A) without cat (a) and cat (b, [cat4+]/[La3+] = 1:0.05) (Experimental conditions: T = 210 °C, t = 4 h, pH = 7), (B) [cat4+]/[La3+] = 1:0.01(c), [cat4+]/[La3+] = 1:0.05(d) and [cat4+]/[La3+] = 1:1 (e) (Experimental conditions: T = 180 °C, t = 24 h) and (C) Corresponding W-H plot.
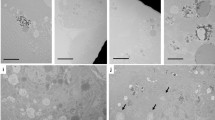
An indication of the morphological features of the nanoparticles was obtained from transmission electron microscopic images (TEM). Gd doped m-LaVO4 (marked as MGL for better representation) nanoparticles were spherical with an average size of around 40 nm (Fig. 4a). In the presence of cat, t-LaVO4, with a rod-like morphology (mean length of 30 nm, mean diameter of 9 nm, the aspect ratio of 3) was obtained (Fig. 4b, marked as TGL for better representation). Morphological changes with varying cat concentration are depicted in Fig. 4c–h, where at 0.01 mM cat (marked as 01GL), nanorods with the length of 20–30 nm and diameter of 7 nm, the aspect ratio of 3 was observed. That the LaVO4 existed in the tetragonal form was confirmed by comparing the lattice fringes (d = 2.2388 Å), with the (301) plane of standard t-LaVO4. At 0.05 mM cat, the nanoparticles (marked as 5GL) existed as irregular rectangular NPs with the length of 14–25 nm and diameter of 12–22 nm respectively. Corresponding high-resolution images and SAED pattern (inset of Fig. 4e) showed the single crystalline character that could easily be correlated to standard t-LaVO4. As the concentration of cat is increased (0.1 mM, marked as 1GL), a thermodynamically stable spherical morphology is obtained, with a diameter in the range of 12 nm. HRTEM image of 1GL, indicated well-defined 2D lattice planes with d spacing of 1.9231 Å indexed to (312) plane [JCPDS-10705226]. SAED (Fig. 4g inset) of 1GL shows a single crystalline diffraction pattern, indexed to (200), (211) and (103) planes of t-LaVO4.
Gd doped LaVO4 nanoparticles obtained by employing cat as a chelating agent was analysed by TGA and FTIR. The spectra presented in Fig. 5 provide an indication of 8–14% organic matter being still present in the residue. TGA profile of nanoparticles showed dehydration of water around 100 °C18, followed by further weight loss between 200–450 °C, corresponding to catechin, as reported elsewhere11. From the FTIR spectrum, it can be seen that the hydroxyl groups in phenolic and water molecules appear as broad absorption band around 3400 cm−1, the doublet bands observed at 1634 and 1410 cm−1 are related to the localized vibration of VO4 groups and C = C stretching frequencies from the aromatic rings. A band around 800 cm−1, corresponds to the characteristic peak of V-O from VO4 groups19 and C-O-C group in catechin molecules7,16.
Dynamic Light Scattering (DLS) in back scattering geometry was performed to determine the hydrodynamic size of the Gadolinium doped LaVO4 nanoparticles. Figure 6a presents the hydrodynamic diameters of the nanoparticles dispersed in double distilled water (1 mg/5 mL). Assuming a spherical geometry, DLS measurement has been carried out. The nanoparticles demonstrated polydisperse behavior (PDI = 0.590) with a number average diameter of 798 nm, attributable to the nonuniform size and nanoparticle aggregation. Number average diameter of nanoparticles in the presence of catechin was low, suggesting that strong capping effect had rendered uniform size. Polydispersity index (PDI) of 0.24, 0.33, 0.27, and 0.27 respectively indicate a near monodisperse distribution.
Zeta potential values provide information on the stability of the nanoparticles in a given environment20,21,22. The pH at which charge of the nanoparticle and its immediate surroundings (double layer) becomes zero (point of zero charge (PZC)) was monitored (Fig. 7A) and it was found that PZC changes from 6.91 to 3.25 and then increases to 7.93. This shift in PZC to a higher pH value could be attributed to the presence of catechin molecules on the nanoparticle surface. In the absence of cat the Gd doped LaVO4 synthesized had a positive charge (28.7 ± 0.5 mV) at pH 8, which shifted to a negative value of −17.1 mV on treatment with cat, indicating that the surface of the nanoparticles was covered with organic moiety7. At cat concentration of 0.05 to 0.1 mM, the zeta potential values of the doped vanadates were more or less constant at around −16 ± 1 mV. A high negative zeta potential, as observed in this study is an indication of the stability of the Gd doped t-LaVO4 making it viable for potential biological applications. A zeta potential value of 1.56 mV observed for the nanoparticles at a cat concentration of 0.01 mM could be attributed to the fact that cationic Ln3+ ions were coordinated to phenolic –OH groups in catechin, resulting in the neutralization of the negative surface charge. The presence of Gd, V, La, O and C was confirmed from the EDAX spectra (Fig. 7B). The atomic ratio for La3+ and Gd3+ was determined as 0.92 and 0.06 respectively, approaching the theoretical value. Gadolinium concentration in MGL, TGL, 01GL, 5GL, 1GL was found to be 2.83, 0.574, 7.712, 10.32, 1.89 mg/kg from ICP-OES measurements.
Luminescence properties of Gd doped t-LaVO4 nanoparticles is shown in Fig. 8. The strong absorption band around 267 nm corresponds to charge transfer from the oxygen ligands to central vanadium metal in VO43− groups23. Gd doped t-LaVO4 exhibit emission peak at 334 nm corresponds to 6P-8S transition of Gd3+ 24 whereas emission group lines between 360 and 520 nm, corresponds to VO43− transitions25. MTT assay26 was performed in order to understand the effect of Gd- doped tetragonal LaVO4 on cell viability and toxicity. The results of MTT assays are given in Supporting Figure S1. The results showed that treatment of HaCaT cells with Gd- doped tetragonal LaVO4 did not affect the viability of the cells. The cells did not show significant toxicity at Gd- doped tetragonal LaVO4 concentration as high as 100 μg. The results are consistent with the microphotographs which revealed that the cell structure and morphology were not affected at a concentration as high as 100 μg (Figure S1).
Magnetization curves measured in the applied magnetic field sweeping from −15 to 15 kG at 300 K, in the presence and absence of cat is presented in Fig. 9. The samples were found to be paramagnetic (P) with a high paramagnetic moment, attributable to a higher number of unpaired electrons in the half-filled 4f7 outermost orbital of the Gd3+ ion27 with Ms value of 42.4, 18.32, 56.19 and 58.87 emu/g respectively. It is known that saturation magnetization of nanoparticles can be affected by structural defects; crystallite size, shape and amount of catechin present on the LaVO4 nanoparticle, i.e. surface state28,29,30,31,32,33. At higher cat, nanoparticles become superparamagnetic (SP), with a low Ms value of 0.0288 emu/g34,35. This shift in Ms may be due to strong co-ordination ability of the catechin molecules36. Comparatively, saturation magnetization on a per-gram basis is lower may be due to the lack of full spin alignment in the particles i.e. spin canting effect induced by the high mass of the nonmagnetic catechin coating on the nanoparticle surface37,38. This clearly establishes that catechin molecules play a key role on magnetic properties of nanoparticles. Table 2 records the coercivity (Hc), saturation magnetization (Ms), remnant magnetization (Mr) and squareness ratio values for the nanoparticles. The ratio of Mr to Ms is almost found to be constant for all paramagnetic material.
Magnetization curves of GL nanoparticles at 300 K 1) without cat (a) and cat (b, [cat4−]/[La3 +] = 1:0.05) (Experimental conditions: T = 210 °C, t = 4 h, pH = 7), 2) [cat4−]/[La3+] = 1:0.01 (c), [cat4−]/[La3+] = 1:0.05 (d) and [cat4−]/[La3+] = 1:1 (e) (Experimental conditions: T = 180 °C, t = 24 h).
We examined the possibility of developing P-Gd and SP-Gd as MRI bimodal contrast agents. To evaluate the MRI imaging properties, series of gadolinium doped LaVO4 nanoparticles in aqueous solutions containing different concentrations (3.6, 1.8, 0.9, 0.45, 0.23 and 0 mM) were prepared for MRI phantom and relaxivity studies. The longitudinal relaxivity (r1) and transverse relaxivity (r2) of the Gd doped t-LaVO4 (with cat) were determined and compared with that of Gd doped m-LaVO4 nanoparticles (without cat) (Fig. 10). It is clear from the Fig. 10 that tetragonal phase had better positive contrast enhancement than that of monoclinic phase. Gd doped t-LaVO4 nanoparticles had an r1 of 0.142 mM−1s−1, which was more than five times that of Gd doped m-LaVO4 nanoparticles (0.030 mM−1s−1). The ratio between transverse and longitudinal relaxivity (r2/r1) was found to be low for Gd doped t-LaVO4 nanoparticles (2.55), compared to that of Gd doped m-LaVO4 nanoparticles (5.2). This increase in the r1 value coupled with a reduction in r2/r1 provides for the Gd doped t-LaVO4 nanoparticles being ideal for use as T1 contrast agent39,40.
In order to understand the role of anisotropic morphology, lanthanum chloride was employed as a precursor at 180 °C for 24 h. The results presented in Fig. 11 and Table 3 indicates a variation in the r2/r1 values. Interestingly at low cat concentration (0.01 mM), nanoparticles exhibited properties ideal for a T2 contrast agents with high r2 value (3.749 mM−1s−1) and r2/r1 of 21.3039,41. At 0.05 mM of catechin, nanoparticles lose their ability as T2 instead to T1 contrast agent with a decrease in the r2/r1 ratio (6.46) and r1 and r2 values are greatly reduced42,43. At a higher cat concentration, SP nanoparticles with almost identical r1 (0.046 mM−1s−1) and r2 (0.068 mM−1s−1) values and moderate r2/r1 (1.48) ratio, with potential to serve as an excellent candidate for T1-T2 dual-mode contrast were obtained. This observation is further supported by phantom imaging studies (Fig. 11)39,44. To conclude, based on the r2/r1 ratio, r1, and r2 values, it has been found that the Gd-doped LaVO4 nanoparticles developed in this study can be tailored to function as T1, T2 and T1-T2 contrast agents through tuning of cat concentration. Such multi-contrast MRI labeling provides unique opportunities for non-invasive multicellular tracking.
Conclusion
In this paper, we have synthesized Gd-doped LaVO4 nanoparticles with different crystal structure and varying morphology, viz., sphere, rods, and irregular rectangular nanocrystals by a catechin directed hydrothermal method. With catechin concentration, the saturation magnetization values of rod shaped Gd-doped LaVO4 was greater than that with spherical shape. During this process, the magnetic properties shifted to superparamagnetism from paramagnetism, owing to catechin strong coordination. The direct result of catechin concentration to magnetic property had a remarkable role in MRI applications. MRI studies established that superparamagnetic Gd-doped LaVO4 could be employed as both T1 and T2 contrast agent, as against the common perspective of the same as a T1 contrast agent alone.
Methods
Synthesis of Gd contrasts with different crystal structure
Gd doped LaVO4 (GL) nanoparticles were prepared by co-precipitation method followed by ligand assisted hydrothermal method, carried out according to previously published methods7. For tetragonal LaVO4 synthesis, we used an efficient ligand- catechin hydrate as a phase transfer agent. 0.06 mmol of catechin hydrate (molar ratio of catechin is 0.05 with respective to La3+ ions) was dissolved in 10 mL of double distilled water, to which molar ratio (1:0.05) of La(NO3)3. 6H2O and Gd (NO3)3. 6H2O aqueous solutions were added in drops and kept stirring for 30 min. To that, 1.2 mmol of the Na3VO4 solution was added in drops resulted in the brown color precipitate. The pH of the brown color precipitate was adjusted to 7. The reaction mixture was autoclaved at 210 °C for 4 h, and the resultant product was washed thrice with water and ethanol twice by centrifugation (1500 rpm for 15 min). It was then air-dried to get the desired product. For monoclinic phase, the same procedure was adopted without catechin hydrate7.
Synthesis of Gd contrasts with varying concentration of catechin hydrate
Different concentrations of catechin hydrate (0.01, 0.05, 0.1 mmol) in 10 mL of distilled water, 1 mmol of LaCl3.7H2O and Gd(NO3)3. 6H2O (molar ratio = 1:0.05) was added in drops and left stirring for 30 min. Then, 1.05 mmol of Na3VO4 solution added in drops and stirred for 10 mins to get a brown color precipitate. The resulting solution undergoes hydrothermal treatment at 180 °C for 24 h followed by centrifugation (1500 rpm for 15 min) with double distilled water thrice and twice with ethanol. The final product was obtained by air drying.
The slow-scan powder XRD data for five Gd doped LaVO4 nanoparticles, were collected with a step size of 0.01° in the 2θ range of 10–80°. The GSAS-EXPGUI58 program was used for the Rietveld structure refinement from the powder XRD data. The refined parameters were scale factor, background as Chebyshev polynomial, unit cell parameters, profile function (Gaussian and Lorentzian parameters, sample displacement) and atomic positions. The initial structural models for five Gd doped LaVO4, were based on their single crystal X-ray structures. The single crystal X-ray structure of Monoclinic LaVO4 was used as a structure model for MGL. The single crystal X-ray structure of Tetragonal LaVO4 was used as a structure model for TGL, 01GL, 5GL, and 1GL. The structural models turned out to be the correct ones in all cases. For all atoms, the isotropic thermal parameters from the single crystal X-ray structure were used and not refined. Positional parameters and profile functions were refined in alternate cycles until no substantial changes were observed in the positional parameters. The structure refinement proceeded smoothly to yield acceptable agreement factors.
Lattice strain was calculated by Williamson-Hall (W-H) method45. A positive slope denotes tensile strain, and a negative slope of the W-H plot denotes compressive strain. A very low lattice strain observed owing to the effective ionic radii mismatch between La3+ and Gd3+ ions.
Measurement of magnetic resonance relaxivities
MR relaxivities of GL nanoparticles were measured using a clinical 1.5 T MR scanner (MAGNETOM Avento Tim System, M/s. Siemens, Germany) equipped with a head coil. For this, phantoms of different concentration of GL (0–3.6 mM) were prepared in deionized water and used. For T2 relaxometry calculations, a modified T2 relaxometry spin echo sequence with TE varying from 15–120 ms with Repetition Time (TR) of 2000 ms were run at three different planes of the phantoms and the pixel intensity with respect to concentration extracted. From the pixel intensity output, the transverse relaxation for each concentration was calculated by employing a linear fit program. For T1 measurements, an inversion-recovery sequence was used with 7 non-equidistant time delays of 50, 100, 300, 700, 1200, 2000 and 3000 ms between inversion and the first 90° excitation pulse. Time of Echo (TE) and Time of Repetition (TR) are chosen as 15 and 4000 ms respectively. From the MR images corresponding to these inversion times, signal intensities for all the T1 were obtained. The T1 relaxation time of each sample was calculated applying these data to the intensity function of the MR signal.
Additional Information
How to cite this article: Vairapperumal, T. et al. Catechin tuned magnetism of Gd-doped orthovanadate through morphology as T1-T2 MRI contrast agents. Sci. Rep. 6, 34976; doi: 10.1038/srep34976 (2016).
References
Nunez, N. O. et al. Surface modified Eu:GdVO4 nanocrystals for optical and MRI imaging. Dalton Transactions 42, 10725–10734, 10.1039/C3DT50676B (2013).
Wang, H. & Wang, L. One-Pot Syntheses and Cell Imaging Applications of Poly(amino acid) Coated LaVO4:Eu3+ Luminescent Nanocrystals. Inorganic Chemistry 52, 2439–2445, 10.1021/ic302297u (2013).
Wang, F., Peng, E., Zheng, B., Li, S. F. Y. & Xue, J. M. Synthesis of Water-Dispersible Gd2O3/GO Nanocomposites with Enhanced MRI T1 Relaxivity. The Journal of Physical Chemistry C 119, 23735–23742, 10.1021/acs.jpcc.5b06037 (2015).
Abdesselem, M. et al. Multifunctional Rare-Earth Vanadate Nanoparticles: Luminescent Labels, Oxidant Sensors, and MRI Contrast Agents. ACS Nano 8, 11126–11137, 10.1021/nn504170x (2014).
Yang, D. et al. Current advances in lanthanide ion (Ln3+)-based upconversion nanomaterials for drug delivery. Chemical Society Reviews 44, 1416–1448, 10.1039/C4CS00155A (2015).
Rocío, C.-V., Carlos, Z. & Concepción, C. Enhanced upconversion multicolor and white light luminescence in SiO 2 -coated lanthanide-doped GdVO 4 hydrothermal nanocrystals. Nanotechnology 23, 505205, 10.1088/0957-4484/23/50/505205 (2012).
Tamilmani, V., Sreeram, K. J. & Nair, B. U. Tuned synthesis of doped rare-earth orthovanadates for enhanced luminescence. RSC Advances 4, 4260–4268, 10.1039/C3RA44979C (2014).
Jia, C.-J. et al. Selective Synthesis of Monazite- and Zircon-type LaVO4 Nanocrystals. The Journal of Physical Chemistry B 109, 3284–3290, 10.1021/jp045967u (2005).
Okram, R., Yaiphaba, N., Ningthoujam, R. S. & Singh, N. R. Is Higher Ratio of Monoclinic to Tetragonal in LaVO4 a Better Luminescence Host? Redispersion and Polymer Film Formation. Inorganic Chemistry 53, 7204–7213, 10.1021/ic500828s (2014).
Zhang, F., Li, G., Zhang, W. & Yan, Y. L. Phase-Dependent Enhancement of the Green-Emitting Upconversion Fluorescence in LaVO4:Yb3+, Er3+. Inorganic Chemistry 54, 7325–7334, 10.1021/acs.inorgchem.5b00851 (2015).
Tamilmani, V., Sreeram, K. J. & Nair, B. U. Catechin assisted phase and shape selection for luminescent LaVO4 zircon. RSC Advances 5, 82513–82523, 10.1039/C5RA17800B (2015).
Raab, T. et al. Catechin Glucosides: Occurrence, Synthesis, and Stability. Journal of Agricultural and Food Chemistry 58, 2138–2149, 10.1021/jf9034095 (2010).
Iñiguez-Franco, F. et al. Antioxidant Activity and Diffusion of Catechin and Epicatechin from Antioxidant Active Films Made of Poly(l-lactic acid). Journal of Agricultural and Food Chemistry 60, 6515–6523, 10.1021/jf300668u (2012).
You, J., Luo, Y. & Wu, J. Conjugation of Ovotransferrin with Catechin Shows Improved Antioxidant Activity. Journal of Agricultural and Food Chemistry 62, 2581–2587, 10.1021/jf405635q (2014).
Botten, D., Fugallo, G., Fraternali, F. & Molteni, C. Structural Properties of Green Tea Catechins. The Journal of Physical Chemistry B 119, 12860–12867, 10.1021/acs.jpcb.5b08737 (2015).
Xiao, L. et al. Enhanced In Vitro and In Vivo Cellular Imaging with Green Tea Coated Water-Soluble Iron Oxide Nanocrystals. ACS Applied Materials & Interfaces 7, 6530–6540, 10.1021/am508404t (2015).
Khokhar, S. & Owusu Apenten, R. K. Iron binding characteristics of phenolic compounds: some tentative structure–activity relations. Food Chemistry 81, 133–140, 10.1016/S0308-8146(02)00394-1 (2003).
Venkateswarlu, S. & Yoon, M. Core–Shell Ferromagnetic Nanorod Based on Amine Polymer Composite (Fe3O4@DAPF) for Fast Removal of Pb(II) from Aqueous Solutions. ACS Applied Materials & Interfaces 7, 25362–25372, 10.1021/acsami.5b07723 (2015).
Yang, L., Li, L., Zhao, M. & Li, G. Size-induced variations in bulk/surface structures and their impact on photoluminescence properties of GdVO4:Eu3+ nanoparticles. Physical Chemistry Chemical Physics 14, 9956–9965, 10.1039/C2CP41136A (2012).
Li, X. et al. Monodisperse Lanthanide Fluoride Nanocrystals: Synthesis and Luminescent Properties. Inorganic Chemistry 51, 3963–3971, 10.1021/ic200925v (2012).
Li, Y., Li, X., Yang, C. & Li, Y. Ligand-Controlling Synthesis and Ordered Assembly of ZnS Nanorods and Nanodots. The Journal of Physical Chemistry B 108, 16002–16011, 10.1021/jp0489018 (2004).
Dubey, S. P., Lahtinen, M. & Sillanpää, M. Tansy fruit mediated greener synthesis of silver and gold nanoparticles. Process Biochemistry 45, 1065–1071, 10.1016/j.procbio.2010.03.024 (2010).
Wu, X. et al. Morphological Control and Luminescent Properties of YVO4:Eu Nanocrystals. The Journal of Physical Chemistry B 110, 15791–15796, 10.1021/jp060527j (2006).
Xu, Z. et al. Self-templated and self-assembled synthesis of nano/microstructures of Gd-based rare-earth compounds: morphology control, magnetic and luminescence properties. Physical Chemistry Chemical Physics 12, 11315–11324, 10.1039/C0CP00169D (2010).
Fan, W., Bu, Y., Song, X., Sun, S. & Zhao, X. Selective Synthesis and Luminescent Properties of Monazite- and Zircon-Type LaVO4:Ln (Ln = Eu, Sm, and Dy) Nanocrystals. Crystal Growth & Design 7, 2361–2366, 10.1021/cg060807o (2007).
Nidhin, M. et al. Fluorescent nanonetworks: A novel bioalley for collagen scaffolds and Tissue Engineering. Scientific Reports 4, 5968, 10.1038/srep05968 (2014).
Werner, E. J., Datta, A., Jocher, C. J. & Raymond, K. N. High-Relaxivity MRI Contrast Agents: Where Coordination Chemistry Meets Medical Imaging. Angewandte Chemie International Edition 47, 8568–8580, 10.1002/anie.200800212 (2008).
Rebodos, R. L. & Vikesland, P. J. Effects of Oxidation on the Magnetization of Nanoparticulate Magnetite. Langmuir 26, 16745–16753, 10.1021/la102461z (2010).
Sun, S. et al. Monodisperse MFe2O4 (M = Fe, Co, Mn) Nanoparticles. Journal of the American Chemical Society 126, 273–279, 10.1021/ja0380852 (2004).
Si, S. et al. Magnetic Monodisperse Fe3O4 Nanoparticles. Crystal Growth & Design 5, 391–393, 10.1021/cg0497905 (2005).
Bao, N., Shen, L., Wang, Y., Padhan, P. & Gupta, A. A Facile Thermolysis Route to Monodisperse Ferrite Nanocrystals. Journal of the American Chemical Society 129, 12374–12375, 10.1021/ja074458d (2007).
Fortin, J.-P. et al. Size-Sorted Anionic Iron Oxide Nanomagnets as Colloidal Mediators for Magnetic Hyperthermia. Journal of the American Chemical Society 129, 2628–2635, 10.1021/ja067457e (2007).
Baaziz, W. et al. Tuning of Synthesis Conditions by Thermal Decomposition toward Core–Shell CoxFe1–xO@CoyFe3–yO4 and CoFe2O4 Nanoparticles with Spherical and Cubic Shapes. Chemistry of Materials 26, 5063–5073, 10.1021/cm502269s (2014).
Kim, B. H., Hackett, M. J., Park, J. & Hyeon, T. Synthesis, Characterization, and Application of Ultrasmall Nanoparticles. Chemistry of Materials 26, 59–71, 10.1021/cm402225z (2014).
Salazar-Alvarez, G. et al. Cubic versus Spherical Magnetic Nanoparticles: The Role of Surface Anisotropy. Journal of the American Chemical Society 130, 13234–13239, 10.1021/ja0768744 (2008).
Walter, A. et al. Mastering the Shape and Composition of Dendronized Iron Oxide Nanoparticles To Tailor Magnetic Resonance Imaging and Hyperthermia. Chemistry of Materials 26, 5252–5264, 10.1021/cm5019025 (2014).
Kolhatkar, A. G., Nekrashevich, I., Litvinov, D., Willson, R. C. & Lee, T. R. Cubic Silica-Coated and Amine-Functionalized FeCo Nanoparticles with High Saturation Magnetization. Chemistry of Materials 25, 1092–1097, 10.1021/cm304111z (2013).
Kim, B. H. et al. Large-Scale Synthesis of Uniform and Extremely Small-Sized Iron Oxide Nanoparticles for High-Resolution T1 Magnetic Resonance Imaging Contrast Agents. Journal of the American Chemical Society 133, 12624–12631, 10.1021/ja203340u (2011).
Dumont, M. F. et al. DNA Surface Modified Gadolinium Phosphate Nanoparticles as MRI Contrast Agents. Bioconjugate Chemistry 23, 951–957, 10.1021/bc200553h (2012).
Zhou, Z. et al. Interplay between Longitudinal and Transverse Contrasts in Fe3O4 Nanoplates with (111) Exposed Surfaces. ACS Nano 8, 7976–7985, 10.1021/nn5038652 (2014).
Joshi, H. M. et al. Effects of Shape and Size of Cobalt Ferrite Nanostructures on Their MRI Contrast and Thermal Activation. The Journal of Physical Chemistry C 113, 17761–17767, 10.1021/jp905776g (2009).
Tong, S., Hou, S., Zheng, Z., Zhou, J. & Bao, G. Coating Optimization of Superparamagnetic Iron Oxide Nanoparticles for High T2 Relaxivity. Nano Letters 10, 4607–4613, 10.1021/nl102623x (2010).
Zeng, J. et al. Anchoring Group Effects of Surface Ligands on Magnetic Properties of Fe3O4 Nanoparticles: Towards High Performance MRI Contrast Agents. Advanced Materials 26, 2694–2698, 10.1002/adma.201304744 (2014).
Huang, G. et al. Tunable T1 and T2 contrast abilities of manganese-engineered iron oxide nanoparticles through size control. Nanoscale 6, 10404–10412, 10.1039/C4NR02680B (2014).
Soni, A. K. & Rai, V. K. Intrinsic optical bistability and frequency upconversion in Tm3+-Yb3+-codoped Y2WO6 phosphor. Dalton Transactions 43, 13563–13570, 10.1039/C4DT01266F (2014).
Acknowledgements
T.V. thanks the DST-Inspire, Govt. of India for the INSPIRE fellowship. A.S. acknowledges CSIR for the Senior Research Fellowship. Authors acknowledge SURE project for their financial support. CLRI Communication Number 1201.
Author information
Authors and Affiliations
Contributions
S.K.J. wrote the main manuscript text. T.V. and A.S. carried out the experiments. J.S.R. and N.B.U. mented the work. All authors reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Vairapperumal, T., Saraswathy, A., Ramapurath, J. et al. Catechin tuned magnetism of Gd-doped orthovanadate through morphology as T1-T2 MRI contrast agents. Sci Rep 6, 34976 (2016). https://doi.org/10.1038/srep34976
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep34976
This article is cited by
-
Concentration-induced morphological changes of biocompatible La3+, Yb3+, Er3+ tri-doped NaYF4 compounds
Applied Physics A (2023)
-
Sensitive detection of extremely small iron oxide nanoparticles in living mice using MP2RAGE with advanced image co-registration
Scientific Reports (2021)
-
Asialoglycoprotein receptor targeted optical and magnetic resonance imaging and therapy of liver fibrosis using pullulan stabilized multi-functional iron oxide nanoprobe
Scientific Reports (2021)
-
Frequency upconversion in catechin assisted \(\hbox {LaF}_{3}\) LaF 3 : \(\hbox {Yb}^{3+}\) Yb 3 + - \(\hbox {Er}^{3+ }\) Er 3 + square nanoplates
Journal of Chemical Sciences (2017)