Abstract
Major depressive disorder (MDD) is the most common neuropsychiatric disease and despite extensive research, its genetic substrate is still not sufficiently understood. The common polymorphism rs6295 of the serotonin-1A receptor gene (HTR1A) is affecting the transcriptional regulation of the 5-HT1A receptor and has been closely linked to MDD. Here, we used positron emission tomography (PET) exploiting advances in data mining and statistics by using machine learning in 62 healthy subjects and 19 patients with MDD, which were scanned with PET using the radioligand [carbonyl-11C]WAY-100635. All the subjects were genotyped for rs6295 and genotype was grouped in GG vs C allele carriers. Mixed model was applied in a ROI-based (region of interest) approach. ROI binding potential (BPND) was divided by dorsal raphe BPND as a specific measure to highlight rs6295 effects (BPDiv). Mixed model produced an interaction effect of ROI and genotype in the patients’ group but no effects in healthy controls. Differences of BPDiv was demonstrated in seven ROIs; parahippocampus, hippocampus, fusiform gyrus, gyrus rectus, supplementary motor area, inferior frontal occipital gyrus and lingual gyrus. For classification of genotype, ‘RandomForest’ and Support Vector Machines were used, however, no model with sufficient predictive capability could be computed. Our results are in line with preclinical data, mouse model knockout studies as well as previous clinical analyses, demonstrating the two-pronged effect of the G allele on 5-HT1A BPND for, we believe, the first time. Future endeavors should address epigenetic effects and allosteric heteroreceptor complexes. Replication in larger samples of MDD patients is necessary to substantiate our findings.
Similar content being viewed by others
Introduction
Major depressive disorder (MDD) is the most common neuropsychiatric disease with a lifetime prevalence of about 16%. Severely limiting all aspects of life and causing fatal outcomes as suicide, it ranks among the most burden-heavy diseases. MDD also poses a threat to health care systems, having caused 3.8% of global disability-adjusted life years (DALY) in 2010. More strikingly, MDD is expected to increase in disability-adjusted life years until 2030, topping the ranking in the developed world.1, 2
Consequently, affective disorders and especially MDD have been studied extensively over the past decades. The decisive role of serotonin (5-HT) has been validated by postmortem, pharmacologic challenge, tryptophan depletion as well as imaging studies.3, 4 Overall, a disequilibrium of 5-HT has been reported for MDD, while the precise etiological mechanisms are still speculative, shrouded by contrarious findings and uncertainty concerning the role of genetics, epigenetics and environmental factors.5, 6, 7, 8, 9 On one hand, the importance of genetic contribution to MDD is universally accepted and twin studies have shown a moderate heritability of about 40%. On the other hand, the role of specific genes and single-nucleotide polymorphisms is still dubious.10 In fact, common polymorphisms might explain only about 0.05% of heritability.11
The serotonin-1A receptor (5-HT1A) is the most important inhibitory receptor of the serotonergic system and has been studied extensively in MDD.12, 13, 14, 15, 16 The 5-HT1A receptor is prevalent in two configurations. Both are inhibitory Gi/Go coupled receptors that mediate their influence through cAMP and calcium channel inhibition. Presynaptical autoreceptors are located in the dorsal and median raphe nuclei of the midbrain, the hive of serotonergic neuronal activity. Serotonergic neurons project to most parts of the brain, exhibiting postsynaptic heteroreceptors active in the cortex, limbic regions, hypothalamus as well as the spinal cord. Overexpressed 5-HT1A autoreceptors and diminished heteroreceptors in MDD have been demonstrated in animal studies, in vivo by PET studies as well as in postmortem studies.17, 18, 19, 20 Thereby, increased inhibition by presynaptic autoreceptors could decrease overall serotonergic activity and contribute to MDD.21 A common hypothesis is that antidepressants require 5-HT1A autoreceptors to be downregulated and desensitized before a treatment effect can be achieved.22, 23, 24, 25 Consequently, as phosphorylation, internalization as well as downregulation are all adaptive mechanisms acting within days, transcriptional effects leading to decreased receptor synthesis remain as a possible explanation for this delay.26
The 5-HT1A receptor gene HTR1A has been one of the most studied candidate genes in MDD. Although many polymorphisms have been considered, most of them are too rare to be of significant relevance or lacked consistency regarding association with MDD and treatment response. The probably most prominent polymorphism linked to MDD is the rs6295 single-nucleotide polymorphism, a common variation at the 1019 site upstream of the basal promoter area, hence also known as C(-1019)G polymorphism.27 The more common C allele of this single-nucleotide polymorphism is recognized as a binding site for the transcriptional factors Deaf1 or NUDR, Hes1 and Hes5.28, 29, 30 On the other hand, the G allele disables binding of the transcriptional factors. These factors repress transcription of 5-HT1A receptors, however, only Deaf1 is also active in mature neuronal cells. More interestingly, Deaf1 shows a divergent effect in presynaptic 5-HT1A autoreceptors of the raphe and postsynaptic heteroreceptors. Deaf1 knockout mice exhibit an increase in transcription of 5-HT1A autoreceptors of about 50%, while heteroreceptors are repressed by up to 30%.31 Based on this solid preclinical foundation, the G allele of rs6295 was associated with a higher occurrence of MDD, bipolar disorder and completed suicide as well as poor response to selective serotonin-reuptake inhibitors.32, 33, 34, 35 Two functional magnetic resonance imaging studies demonstrated altered reactivity of the amygdala of GG carriers with MDD and healthy subjects, respectively, in emotionally valanced faces as well as threat-related stimuli.36, 37 However, some studies also reported opposite results.38, 39, 40
Few PET studies investigated the effect of rs6295 on 5-HT1A binding, showing greater binding of G-allele carriers in the dorsal raphe nuclei of MDD patients, whereas no significant effects were found in other areas.41, 42, 43 However, a replication analysis in a bigger sample by the same group failed to demonstrate any associations.44 In line with the positive results, G-allele carriers have been suggested to show decreased response to treatment in clinical studies. However, another recent PET study linked higher radioligand binding to 5-HT1A receptors in the raphe nuclei to more pronounced treatment response to selective serotonin-reuptake inhibitor, which is conflicting with higher raphe binding reported in G-allele carriers.45
Based on these ambiguous findings, we conducted a PET study using [carbonyl-11C]WAY-100635 to shed more light on the role of rs6295 in MDD. Due to the molecular architecture of this polymorphism, we further hypothesized that the diverse effect of rs6295 on pre- and postsynaptical receptors should be more refined when applying multivariate machine learning tools for classification. New statistical methods and especially machine learning have been implemented in psychiatric research over the last years as they offer advantages over conventional univariate statistics. They are suitable for large data sets with high number of predictors and allow classification by pattern recognition instead of main and interaction effects.46 As both ‘RandomForest’ (RF) and ‘Support Vector Machines’ (SVM) have been shown to produce strong results in classification, we applied these techniques to test our hypothesis that G-allele carriers will show higher binding to [carbonyl-11C]WAY-100635 in the raphe nuclei while showing lower binding in the projection areas.47 We expected a successful distinction of G homozygotes and C carriers based on the PET data even if effects of rs6295 should not be demonstrable with classical statistical approaches.
Materials and methods
Subjects
This is a pooled sample derived from previous studies, however, all genetic data regarding rs6295 are unpublished.48, 49, 50, 51, 52, 53 Eighty-one subjects aged 18–65 years were enrolled in this neuroimaging genetics study with a cross-sectional design. Sixty-two healthy subjects (40 female) and 19 acutely depressed patients (6 female) diagnosed according to Structured Clinical Interview for DSM-IV type disorders (SCID I+II) were included. Severity was assessed for a subsample of patients using the Hamilton Depression Rating Scale (n=5, HAMD: 19.6±3.4, mean±s.d., all ⩾16) and four patients suffered from generalized anxiety disorder as well. Baseline characteristics of the sample can be found in Supplementary Table 1. All the subjects were measured with [carbonyl-11C]WAY-100635 and underwent a thorough physical and neurological examination, assessment of clinical history, ECG, routine laboratory analysis, urinary drug and pregnancy screening. All the subjects were required to be free of any psychotropic medication at least 3 months before enrollment and no severe somatic condition nor other neuropsychiatric diagnose except anxiety disorders were tolerated. Lifetime administration of neuropsychiatric medication was not registered. Written informed consent after detailed oral information concerning all the study procedures was mandatory for all the subjects. The study and all related procedures were approved by the Ethics Committee of the Medical University of Vienna.
Genotyping
Genotyping was performed as previously described.48 Shortly, 9 ml ethylene-diamine-tetraacetic-acid blood samples were collected from each subject and DNA was isolated from whole blood via QiaAmp DNA blood maxi kit (Qiagen, Hilden, Germany). Genotyping was performed using the iPLEX assay on the MassARRAY MALDI‐TOF mass spectrometer as described previously.54 Allele-specific extension products were identified and genotypes allocated by Typer 3.4 Software (Sequenom, San Diego, CA, USA). For genotyping quality criteria, a single-nucleotide polymorphism call rate over 99% was required. Blood samples for genotyping were anonymized to ensure blinding.
Radiochemistry and PET procedures
Radiosynthesis of [carbonyl-11C]WAY-100635 and all scans were performed at the Division of Nuclear Medicine of the Department of Biomedical and Image‐guided Therapy of the Medical University of Vienna. One PET scan (General Electric Medial Systems, Milwaukee, WI, USA) was conducted per subject using the tracer [carbonyl-11C]WAY-100635, which has high affinity and selectivity for the 5-HT1A receptor. For a detailed description of the synthesis, please see ref. 55. Concerning measurement procedures, first a 5 min transmission scan using a retractable 68Ge rod source for tissue attenuation correction was performed. Subsequently, the dynamic emission scan was acquired in three-dimensional mode. Mean injected dose was 312.04±105.84 MBq, specific activity at the time of injection was 285.47±251.22 GBq μmol−1 and radiochemical purity was above 95%. Reconstruction of the data was performed for 35 transaxial sections (128 × 128 matrix) using an iterative filtered back projection algorithm (FORE‐ITER). The spatial resolution was 4.36 mm full‐width at half maximum 1 cm next to the center of the field of view. Magnetic resonance images were acquired from all the participants for co-registration using a 3‐Tesla Philips scanner (Achieva) and three-dimensional T1 FFE‐weighted sequences, yielding 0.88 mm slice thickness and in-plane resolution of 0.8 × 0.8 mm.56
For better image quality, during the PET scans, subjects were placed with their head parallel to the orbitomeatal line guided by a laser beam system to ensure full coverage of the neocortex and the cerebellum in the field of view. A polyurethane cushion and head straps were used to minimize head movement and to guarantee a soft head rest during the whole scanning period.
Data preprocessing
PET preprocessing was done in SPM8 (Wellcome Trust Centre for Neuroimaging, London, UK, http://www.fil.ion.ucl.ac.uk/spm/) as described previously.57 Personnel involved in data preprocessing was blinded to the subjects' genotype or diagnosis. After realignment to the motion-free mean image, scans of the entire time series were summed up and spatially normalized (affine regularization, average-sized template) to a tracer-specific template within standard MNI-space (Montreal Neurological Institute). Thereafter, the resulting transformation matrix was applied to each time frame.
We assessed in vivo target structure density as indexed by 5-HT1A receptor binding potentials (BPND), which represent the ratio at equilibrium of specifically bound radioligand to that of non-displaceable radioligand in tissue.58 All binding potentials were computed using the voxel-wise modeling tool in the PMOD 3.509 software package (PMOD Technologies, Zurich, Switzerland) and applying the two-parameter linearized reference tissue model (MRTM2).15
We modeled 5-HT1A BPND as previously described by our group using the insula as receptor-rich region and the cerebellum as receptor-poor region.15 The cerebellar gray matter excluding cerebellar vermis and venous sinus served as reference region. Mean overall BPND and mean cerebellar BP are listed in Supplementary Table 1. Regions of interest (ROI) were taken from an automated anatomical labeling-based atlas after normalization of BPND maps to standard MNI-space, except for the dorsal raphe nuclei (DRN), which were located manually in PMOD due to known difficulties of automated detection for this ROI.59 The values were averaged across both hemispheres. Due to inherent smoothness of PET data of the scanner and temporary smoothing during normalization, we did not smooth during statistical processing.
Statistical analysis
All statistics were performed using the statistical software ‘R 3.3.3’ (cran.r-project.org). Analyses were performed for the combined sample of 19 patients and 62 healthy controls with group considered as a factor for all models. If group showed a significant effect, models were also generated for the subgroups of healthy and MDD subjects only. Concerning genotype, GG allele carriers were compared with CC and CG allele carriers to maximize the sensitivity for the region-specific alterations in binding potential. Based on previous research, we expected G homozygotes to be affected by transcriptional dysregulation comparable to knockout studies of Deaf1.31 Furthermore, we divided all normalized BPND values with BPND of the DRN, resulting in a value further referenced as BPDiv. As the DRN is expected to show opposite influence from rs6295 than all other ROIs, this measure was undertaken to increase sensitivity for effects in projection areas, which have not been demonstrable in previous PET studies. An additional rationale behind this procedure that we had successfully applied for a prediction model before was to get rid of interpersonal variance in 5-HT1A receptor binding, which could confound genotype effects.51
Differences of 5-HT1A BPDiv between HTR1A C(-1019)G genotype were computed using a ROI approach. Differences between genotype groups (GG vs C carriers) were calculated with a linear mixed model as provided by the ‘lmne’ package of ‘R’.60 Thereby, subject (between-subject factor) and ROI (within-subject factor) served as the random factors and HTR1A rs6295 genotype status, group, ROI, age and sex served as fixed factors. In total, 46 ROIs were integrated in the model. Significance was determined by P<0.05, Bonferroni corrected. Based on these results, post hoc analyses were performed for all ROIs to further specify significant mixed-model effects. For post hoc analysis, a P-value threshold of 0.05 was determined.
Furthermore, we conducted a machine-learning classification using the ‘randomForest’ (RF) and ‘e1071’ (SVM) package for the statistical software ‘R’.61 For machine-learning classification, a ROI-based and a voxel-wise model were computed.
RF assigns importance values to the predictors based on their usefulness for the classification model, disregarding classical main or interaction effects. Thereby, the most helpful variables for classification of the genotype show the highest importance values measured by mean decrease in Gini. No power calculation for RF has been established so far, however, recent investigations point toward sufficient reliability even with the number of predictors outreaching the observations, with limitations regarding sufficient patient counts and missing data.46, 47 For the ROI approach, BPDiv was used for classification. The voxel-wise approach, however, would surpass the computational capabilities of the machine-learning algorithm. To reduce the number of features without a priori selection, we decided to increase the voxel ranges from 2 × 2 × 2 mm to 4 × 4 × 4 mm, thereby reducing the number of predictors to 18 050. To address interpersonal variation in 5-HT1A binding for this approach, we normalized BPND values for all 18 050 voxels by transforming them to values within a range from 0 to 1, thereby attributing 1 to the highest and 0 to the lowest BPND of a specific subject. Finally, after variable importance was calculated, the subjects were divided into training and test sets for the prediction of genotype based on BPND using a 10-fold cross-validation design as implemented in ‘rfcv’ function of ‘randomForest’.
RF was chosen as the primary algorithm for this analysis as we expected highest variable importance in the DRN. Importance measurements might therefore be a useful alternative for automated or manual labeling of the DRN. However, as there is no clear recommendation as to which specific machine-learning algorithm to use for imaging genetics, we decided to also compute an exploratory model using SVM as implemented in the ‘R’ package ‘e1071’.62 Similar to RF, SVM is a machine-learning algorithm fit for classification and regression. Based on the construction of a discriminative hyperplane in high-dimensional space, successful classification depends on functional margin and generalization error. The implemented function ‘tune.svm’ was used to determine the hyperparameters cost and gamma, regulating bias and variance based on error penalty and the nonlinear kernel function. The optimal model was chosen from a range of 2−10 to 1 for gamma and 2−1 to 10 for cost. Other parameters were kept at default settings. A voxel-wise (normalized BPND) and ROI-based approach (BPDiv) similar to the RF analysis was conducted.
For the machine-learning analyses, separate models were computed for the patient and the control groups, as well as the combined sample.
Results
The patients’ sample showed lower age by an average of 7 years compared with the healthy subjects (P=0.666) and more women were featured in the patients’ group (P=0.037). Allelotypes for rs6295 were in Hardy–Weinberg equilibrium for healthy as well as depressed subjects (P<1). The G-allele was equally distributed in both the groups. For details on demographic parameters, please see also the Supplementary Section.
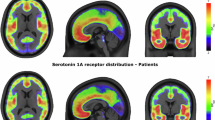
As can be seen in Figure 1, the DRN feature a higher average BPND in GG allelotype than in C allele carriers, even more so in the patients’ group. Other ROIs show rather similar appearance of BPND for rs6295 genotype. Overall, the patient group exhibits slightly diminished BPND (mean BPND 2.53±0.74 for patients vs 2.71±0.7 for controls). For the patients’ group, a graphic representation of BPDiv for each ROI can be found in Figure 2; for a boxplot of all ROIs and a table of mean BPND and BPDiv according to group and genotype, please see the Supplementary Section.
Boxplot for binding potential (BPND) for the dorsal raphe nuclei, showing BPND on the y axis. On the left, BPND for the healthy controls (n=62) is portrayed. On the right side, BPND of the patients subgroup (n=19) is shown. BPND is portrayed for merged CC and CG carriers as well as GG homozygotes to maximize the effect of the rs6295 polymorphism. Mean values are provided for each group and genotype. The difference in DRN BPND did not reach statistical significance. DRN, dorsal raphe nuclei.
Average binding potential (BPND) divided by BPND of the dorsal raphe ROI (BPDiv) for the patients’ group (n=19). The color bar represents BPDiv values ranging from 0 (blue) to 2 (red). G allele homozygotes (n=4) are compared with the merged sample of C allele homozygotes and CG heterozygotes (n=15). G allele homozygotes suffering from major depressive disorder show overall lower BPDiv.
Mixed-model results
As expected, ROI showed significant effects in all mixed models (P<0.001, corrected; F=641.663 overall and 149.134 for the patients’ subgroup). Furthermore, a three-way interaction effect could be demonstrated for ROI, group and genotype (P=0.019, corrected; F=1.482) as shown in Table 1, section A. No main or two-way interaction effect were found for group and genotype. Repeating the mixed model in the patients’ and healthy group separately yielded no effect of genotype in healthy subjects. For the patients’ group, no main effect but a significant two-way interaction effect between ROI and genotype was found (P=0.017, corrected; F=1.511). Please consider also Table 1, section B.
Further tracking this effect down, post hoc analyses produced differences between GG and C allele carriers in seven regions, also portrayed in Table 1, Section C: the fusiform gyrus (P=0.041; F=4.920), gyrus rectus (P=0.048; F=4.543), hippocampus (P=0.046; F=4.609), inferior occipital gyrus (P=0.044; F=4.718), parahippocampus (P=0.045; F=4.679), lingual gyrus (P=0.027; F=5.849) and supplementary motor area (P=0.049; F=4.468). Thereby, GG carriers showed diminished BPDiv in these regions, suggesting higher BPND in the DRN and reduced BPND in the projection areas. These effects are portrayed in Figure 3.
Boxplot showing the average binding potential (BPND) divided by BPND of the dorsal raphe region of interest (ROI; BPDiv) for the patients’ group (n=19). On the x axis, ROIs reaching significance in post hoc analysis of variance (ANOVA) are shown, the y axis shows binding potential BPDiv. G allele homozygotes are colored yellow, C allele carriers red. P-values of the post hoc ANOVA (uncorrected) are shown for all ROI.
Machine-learning results
RF reached an accuracy around 0.725 for all samples (vs 0.750 for SVM) for classification of genotype regardless of voxel or ROI-based approach. The predictive power was severely limited by a sensitivity of only 0.2 (vs 0.1 for SVM). Therefore, no useful prediction of genotype could be achieved either with RF or SVM. For a comprehensive table of classification parameters, please see Table 2.
Discussion
Applying conventional mixed model and more advanced machine-learning algorithms, namely RF and SVM, in a large sample of 62 healthy subjects and 19 MDD patients studied with PET and [carbonyl-11C]WAY-100635, an effect of HTR1A rs6295 genotype on BPDiv could be observed in the MDD group in seven ROI.
In contrast to many other polymorphisms, rs6295 has been extensively studied and the molecular mechanics of the C(-1019)G variation have been described in detail.21, 29 Deaf1 transcription factor is only available to the C but not G allele at the transcription site, resulting in a vastly diminished binding in GG carriers. As Deaf1 has locally divergent effects, with increased 5-HT1A heteroreceptor activity in the serotonergic projection areas and decreased 5-HT1A autoreceptors activity in the raphe nuclei, a robust effect of rs6295 carrier status should be demonstrable in PET imaging. Thereby, the divergent direction of 5-HT1A manipulation in raphe ROI compared with other areas would be expected to highlight this effect despite interpersonal variation in 5-HT1A binding, which has been shown to be a significant limitation in our previous studies.
Nevertheless, only differences in the DRN have been recognized by the two previous PET studies investigating rs6295 in MDD. A study published in 2006 suggested that DRN BPF would increase with the number of G alleles and this finding was later confirmed in a second sample by the same group in 2012.41, 43 However, as in a recent replication study of the same group, no effect of rs6295 could be observed, they concluded that this polymorphism most likely does not affect BPF considered separately.44 Another study in depressed bipolar patients found higher BPF in amygdala and hippocampus as well as DRN in G allele carriers.63 Interestingly, the results of PET studies on 5-HT1A seem to be dependent on the BP parameter investigated. BPF has repeatedly been shown to be raised in MDD patients, whereas binding potential non-displaceable (PBND) usually was found diminished.17, 41, 42, 43, 44, 64, 65, 66 This has been explained by differences in reference region binding that impact BPND. Convergent with these positive PET findings in MDD, but divergent from the molecular fundamentals, we also did not detect significant differences in BPND in any serotonin projection areas. However, although not significant, differences in BPND have been more distinct in the MDD group, featuring a higher average BPND in the DRN of GG carriers as we had expected.
As has been suggested by a recent review on rs6295 in MDD, trait effects in healthy subjects have been lacking except for an association of impulsivity with the G allele and increased negative emotionality in a reward-punishment paradigm.21 On the other hand, the G allele has been shown to be overexpressed in MDD, bipolar disorder and suicide victims, to modify response to selective serotonin-reuptake inhibitor in depressed patients and to alter reactions to various paradigms in functional magnetic resonance imaging. Recent findings in mouse models suggested a stress-mediated control of Deaf1 activity, which was shown to be decreased in chronically stress-exposed mice.67 In conformity, the GG allelotype was indicated to stunt glucocorticoid response to stress and lead to overall susceptibility to stress in MDD and anxiety patients. In synopsis, these data suggest an important lifetime contribution of rs6295 to serotonin equilibrium and risk for mood disorders. Although these effects may be present in healthy subjects as well, they seem to be mostly relevant and visible in vivo after transition to patient status, most likely triggered by stress and negative life events. Therefore, it seems likely that the region-specific effect initially reported by the group of Lemonde, Czesak and Albert can only be observed in patients.27, 30 Difficulties to track these effects down in a clinical sample might derive from compensational regulation of 5-HT, increasingly so as in Deaf1 knockout mice a more prominent role of other factors as Pet and Freud1 and 2 that are usually overshadowed by Deaf1 has been reported by the same group.27, 31
Based on the overall encouraging but still incongruous results, we decided to apply the ratio of ROI/DRN as alternative measure BPDiv. The ratio of serotonin core to projection areas has been successfully fielded before by our group, providing a better predictor for treatment response to selective serotonin-reuptake inhibitor than original BPND.51 Here, we could show that GG carriers suffering from MDD displayed lower BPDiv in seven ROI, including 5-HT1A mainstays as the hippocampus and parahippocampus as well as the fusiform gyrus, gyrus rectus, inferior occipital gyrus, lingual gyrus and supplementary motor area. Thereby, our results are conformable to the original proposal of Albert and colleagues, both demonstrating a higher BP in DRN and lower BP in cortical and projection areas, meaning lower BPDiv in GG allelotype carriers.21 As we did not find significant effects of rs6295 on BPND but on BPDiv, this methodological difference might also explain negative findings by previous investigations.44
However, while our results clearly support previous findings from in vitro, in vivo animal and human samples, the question remains how a seemingly distinct connection as the rs6295 polymorphism in MDD can produce divergent results as shown in the PET studies performed so far on that topic. Even more surprisingly, the multivariate machine learning approach failed at deriving a classification model for allelotypes of rs6295. Multivariate analyses as RF and SVM should be able to translate the suspected divergent changes in BPND or BPDiv in an accurate classification of GG and C allele carriers. Given the significant results of our mixed-model analysis, the effects attributable to genotype might still be too delicate to enable successful engagement of a predictive algorithm. Arguing that effects could only be observed in the patient sample, only 19 subjects were disposable for RF and SVM, limiting the effectiveness of these algorithms. Also, only four patients exhibiting the GG alellotype were featured, which might be too low of a number to guarantee stable classification with high numbers of predictors. On the other hand, we still achieved an accuracy above random guessing. As importance measurement by RF could potentially provide an alternative labeling method for the DRN, we advocate further research applying multivariate techniques as there is certainly a potential for future refinement.
Besides the already mentioned compensational effects that can be expected to bias results, other factors should be taken into account as well. Most importantly to our concern, epigenetic contribution as methylation status has been widely neglected so far in imaging genetics in MDD. Epigenetic variation is a distinctive feature in monozygotic twins who show discordant affection from MDD and several methylation markers were associated with MDD.68, 69 Also, methylation-dependent 5-HT1A receptor upregulation was recently constituted, for example, mediated through an Sp4 site prone to stress-induced hypermethylation.70 As Deaf1 activity has been shown to correlate with stress and life events, the C(-1019)G binding site might be inactive in some subjects, therefore resulting in erroneous grouping of genotype. Recent reviews in the field of genetics in MDD have strongly recommended to check for methylation effects to fathom the rampant ambiguity of association findings.71, 72
Furthermore, as the compensational capability of the neurotransmitter system has been addressed before, the still fresh area of allosteric heteroreceptor complexes might be of relevance.73 Regarding 5-HT1A receptors, brain-derived neurotrophic factor and galanin receptor heteroreceptor complexes have been highlighted as possible key targets of MDD as well as therapeutic agents. Especially brain-derived neurotrophic factor–5-HT1A autoreceptor complexes in the raphe nuclei might be of paramount importance for serotonin equilibrium as the negative feedback of autoreceptors might be absorbed in a trophic boost effect.74, 75 Interaction effects of such complexes are hardly understood at this point, therefore possibly disguising results of single receptor approaches. Regarding recent methodological advances in PET imaging that will possibly cut short scanning time significantly by relying on a bolus/constant infusion technique, multi-receptor imaging studies could pave the way to a clearer understanding of the role of 5-HT1A receptors and rs6265.76, 77
Except for these considerations, some clear limitations of our study must be discussed. First, our sample comprises different study populations, resulting in different ratios of sex and age for MDD and healthy subject subgroups as no matching for these variables could be performed. Due to the resource-intensive nature of PET studies, this synoptic approach was necessary to gain a sufficiently large sample for our analyses. Most important, however, the patients’ sample is featuring four subjects among the CG heterozygotes suffering from a comorbid anxiety disorder. As involvement of 5-HT1A receptors has been shown to differ between anxiety disorders and MDD, also regarding the rs6295 polymorphism and the Hes1 and 5 transcription factors, this comorbidity might impede interpretation of our results. A recent review argued for elevated 5-HT1A levels in adult anxiety opposed to low 5-HT1A levels in MDD, pointing towards an early affection of low 5-HT in neonatal anxiety, related to Hes1 and 5, which are mostly active at this time of neuronal development.78 On the other hand, one of the studies focused on rs6295 in MDD and anxiety suggested strongest associations in comorbid anxiety and depressive disorder.79 Furthermore, while all subjects featured for this analysis were free of neuropsychiatric medication for at least 3 months before scanning, lifetime records concerning drug naivety were not available. Consequently, we cannot rule out bias of our results caused by previous medication. This might be of special importance as previous studies have argued that 5-HT1A receptor binding is significantly impacted by previous medication up to at least 4 years.43 Also, depression severity and sociodemographic or other clinical predictors could not be implemented in this analysis as they were not registered for most of the subjects. Hence, we cannot rule out that differences in severity or heterogeneity in sociodemographic parameters impacts our results.
Furthermore, even though we conducted this study featuring one of the largest samples regarding PET imaging genetics in MDD, only 19 patients were disposable for analysis. Although we could track down genotype effects using BPDiv, statistically no significant elevation of BPND in the raphe nuclei of GG carriers opposed to reduced BPND in projection areas could be observed for the patients’ subgroup. Therefore, more resounding results could be expected in a larger cohort, benefitting from higher power. This might be even more relevant for machine-learning classification, which failed to provide useful prediction in this study. Even though RF allegedly works for samples with observations largely outnumbered by predictors, this robustness is supposedly only guaranteed with sufficient overall sample size.47, 80
Considering these limitations, we cannot rule out considerable bias by comorbidity, demographics, differing severity or lifetime medication. On the other hand, this study introduces the application of BPDiv to limit some of the known issues with imaging genetics as interpersonal variance. Our results suggest that refined methodical and statistical arrangements can enhance detection of complex effects. Furthermore, even though no successful application could be performed in this study, we also believe that machine learning holds great potential that we adumbrated by our rationale and findings. Keeping in mind the limitations, we provide further evidence for the important role of the rs6295 polymorphism in affective disorders using PET imaging and [carbonyl-11C]WAY-100635. Our results are overall in line with preclinical data, mouse model knockout studies as well as previous clinical analyses, demonstrating the two-pronged effect of the G allele on 5-HT1A BPDiv for, we believe, the first time. Future endeavors should also address epigenetic effects and allosteric heteroreceptor complexes, possibly by scanning for multiple targets, and replication in larger samples of MDD patients is necessary to further substantiate our findings.
References
Whiteford HA, Degenhardt L, Rehm J, Baxter AJ, Ferrari AJ, Erskine HE et al. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet 2013; 382: 1575–1586.
Whiteford HA, Ferrari AJ, Degenhardt L, Feigin V, Vos T . The global burden of mental, neurological and substance use disorders: an analysis from the Global Burden of Disease Study 2010. PLoS ONE 2015; 10: e0116820.
Young SN . Acute tryptophan depletion in humans: a review of theoretical, practical and ethical aspects. J Psychiatry Neurosci 2013; 38: 294–305.
Cheetham SC, Crompton MR, Katona CL, Horton RW . Brain 5-HT1 binding sites in depressed suicides. Psychopharmacology (Berl) 1990; 102: 544–548.
Scarr E, Millan MJ, Bahn S, Bertolino A, Turck CW, Kapur S et al. Biomarkers for Psychiatry: The Journey from Fantasy to Fact, a Report of the 2013 CINP Think Tank. Int J Neuropsychopharmacol 2015; 18: pyv042.
Porcelli S, Drago A, Fabbri C, Gibiino S, Calati R, Serretti A . Pharmacogenetics of antidepressant response. J Psychiatry Neurosci 2011; 36: 87–113.
Young JJ, Silber T, Bruno D, Galatzer-Levy IR, Pomara N, Marmar CR . Is there Progress? An overview of selecting biomarker candidates for major depressive disorder. Front Psychiatry 2016; 7: 72.
Kaufman J, DeLorenzo C, Choudhury S, Parsey RV . The 5-HT1A receptor in major depressive disorder. Eur Neuropsychopharmacol 2016; 26: 397–410.
Gryglewski G, Lanzenberger R, Kranz GS, Cumming P . Meta-analysis of molecular imaging of serotonin transporters in major depression. J Cereb Blood Flow Metab 2014; 34: 1096–1103.
Sullivan PF, Neale MC, Kendler KS . Genetic epidemiology of major depression: review and meta-analysis. Am J Psychiatry 2000; 157: 1552–1562.
Gratten J, Wray NR, Keller MC, Visscher PM . Large-scale genomics unveils the genetic architecture of psychiatric disorders. Nat Neurosci 2014; 17: 782–790.
Saulin A, Savli M, Lanzenberger R . Serotonin and molecular neuroimaging in humans using PET. Amino Acids 2012; 42: 2039–2057.
Fink M, Wadsak W, Savli M, Stein P, Moser U, Hahn A et al. Lateralization of the serotonin-1A receptor distribution in language areas revealed by PET. Neuroimage 2009; 45: 598–605.
Hoflich A, Baldinger P, Savli M, Lanzenberger R, Kasper S . Imaging treatment effects in depression. Rev Neurosci 2012; 23: 227–252.
Savli M, Bauer A, Mitterhauser M, Ding YS, Hahn A, Kroll T et al. Normative database of the serotonergic system in healthy subjects using multi-tracer PET. Neuroimage 2012; 63: 447–459.
Stein P, Savli M, Wadsak W, Mitterhauser M, Fink M, Spindelegger C et al. The serotonin-1A receptor distribution in healthy men and women measured by PET and [carbonyl-11C]WAY-100635. Eur J Nucl Med Mol Imaging 2008; 35: 2159–2168.
Parsey RV, Ogden RT, Miller JM, Tin A, Hesselgrave N, Goldstein E et al. Higher serotonin 1A binding in a second major depression cohort: modeling and reference region considerations. Biol Psychiatry 2010; 68: 170–178.
Miller JM, Brennan KG, Ogden TR, Oquendo MA, Sullivan GM, Mann JJ et al. Elevated serotonin 1A binding in remitted major depressive disorder: evidence for a trait biological abnormality. Neuropsychopharmacology 2009; 34: 2275–2284.
Arango V, Underwood MD, Boldrini M, Tamir H, Kassir SA, Hsiung S et al. Serotonin 1A receptors, serotonin transporter binding and serotonin transporter mRNA expression in the brainstem of depressed suicide victims. Neuropsychopharmacology 2001; 25: 892–903.
Stockmeier CA, Shapiro LA, Dilley GE, Kolli TN, Friedman L, Rajkowska G . Increase in serotonin-1A autoreceptors in the midbrain of suicide victims with major depression-postmortem evidence for decreased serotonin activity. J Neurosci 1998; 18: 7394–7401.
Albert PR, Fiori LM . Transcriptional dys-regulation in anxiety and major depression: 5-HT1A gene promoter architecture as a therapeutic opportunity. Curr Pharm Des 2014; 20: 3738–3750.
Hjorth S, Auerbach SB . Further evidence for the importance of 5-HT1A autoreceptors in the action of selective serotonin reuptake inhibitors. Eur J Pharmacol 1994; 260: 251–255.
Hjorth S, Bengtsson HJ, Milano S . Raphe 5-HT1A autoreceptors, but not postsynaptic 5-HT1A receptors or beta-adrenoceptors, restrain the citalopram-induced increase in extracellular 5-hydroxytryptamine in vivo. Eur J Pharmacol 1996; 316: 43–47.
Romero L, Artigas F . Preferential potentiation of the effects of serotonin uptake inhibitors by 5-HT1A receptor antagonists in the dorsal raphe pathway: role of somatodendritic autoreceptors. J Neurochem 1997; 68: 2593–2603.
Liu RJ, Lambe EK, Aghajanian GK . Somatodendritic autoreceptor regulation of serotonergic neurons: dependence on L-tryptophan and tryptophan hydroxylase-activating kinases. Eur J Neurosci 2005; 21: 945–958.
Richardson-Jones JW, Craige CP, Guiard BP, Stephen A, Metzger KL, Kung HF et al. 5-HT1A autoreceptor levels determine vulnerability to stress and response to antidepressants. Neuron 2010; 65: 40–52.
Lemonde S, Turecki G, Bakish D, Du L, Hrdina PD, Bown CD et al. Impaired repression at a 5-hydroxytryptamine 1A receptor gene polymorphism associated with major depression and suicide. J Neurosci 2003; 23: 8788–8799.
Adeosun SO, Albert PR, Austin MC, Iyo AH . 17beta-estradiol-induced regulation of the novel 5-HT1A-related transcription factors NUDR and Freud-1 in SH SY5Y cells. Cell Mol Neurobiol 2012; 32: 517–521.
Albert PR . Transcriptional regulation of the 5-HT1A receptor: implications for mental illness. Philos Trans R Soc Lond B Biol Sci 2012; 367: 2402–2415.
Czesak M, Lemonde S, Peterson EA, Rogaeva A, Albert PR . Cell-specific repressor or enhancer activities of Deaf-1 at a serotonin 1A receptor gene polymorphism. J Neurosci 2006; 26: 1864–1871.
Czesak M, Le Francois B, Millar AM, Deria M, Daigle M, Visvader JE et al. Increased serotonin-1A (5-HT1A) autoreceptor expression and reduced raphe serotonin levels in deformed epidermal autoregulatory factor-1 (Deaf-1) gene knock-out mice. J Biol Chem 2012; 287: 6615–6627.
Donaldson ZR, le Francois B, Santos TL, Almli LM, Boldrini M, Champagne FA et al. The functional serotonin 1a receptor promoter polymorphism, rs6295, is associated with psychiatric illness and differences in transcription. Transl Psychiatry 2016; 6: e746.
Lemonde S, Du L, Bakish D, Hrdina P, Albert PR . Association of the C(-1019)G 5-HT1A functional promoter polymorphism with antidepressant response. Int J Neuropsychopharmacol 2004; 7: 501–506.
Serretti A, Artioli P, Lorenzi C, Pirovano A, Tubazio V, Zanardi R . The C(-1019)G polymorphism of the 5-HT1A gene promoter and antidepressant response in mood disorders: preliminary findings. Int J Neuropsychopharmacol 2004; 7: 453–460.
Villafuerte SM, Vallabhaneni K, Sliwerska E, McMahon FJ, Young EA, Burmeister M . SSRI response in depression may be influenced by SNPs in HTR1B and HTR1A. Psychiatr Genet 2009; 19: 281–291.
Dannlowski U, Ohrmann P, Bauer J, Kugel H, Arolt V, Heindel W et al. Amygdala reactivity to masked negative faces is associated with automatic judgmental bias in major depression: a 3T fMRI study. J Psychiatry Neurosci 2007; 32: 423–429.
Fakra E, Hyde LW, Gorka A, Fisher PM, Munoz KE, Kimak M et al. Effects of HTR1A C(-1019)G on amygdala reactivity and trait anxiety. Arch Gen Psychiatry 2009; 66: 33–40.
Huang YY, Battistuzzi C, Oquendo MA, Harkavy-Friedman J, Greenhill L, Zalsman G et al. Human 5-HT1A receptor C(-1019)G polymorphism and psychopathology. Int J Neuropsychopharmacol 2004; 7: 441–451.
Hettema JM, An SS, van den Oord EJ, Neale MC, Kendler KS, Chen X . Association study between the serotonin 1A receptor (HTR1A) gene and neuroticism, major depression, and anxiety disorders. Am J Med Genet B Neuropsychiatr Genet 2008; 147B: 661–666.
David SP, Murthy NV, Rabiner EA, Munafo MR, Johnstone EC, Jacob R et al. A functional genetic variation of the serotonin (5-HT) transporter affects 5-HT1A receptor binding in humans. J Neurosci 2005; 25: 2586–2590.
Hesselgrave N, Parsey RV . Imaging the serotonin 1A receptor using [11C]WAY100635 in healthy controls and major depression. Philos Trans R Soc Lond B Biol Sci 2013; 368: 20120004.
Parsey RV, Olvet DM, Oquendo MA, Huang YY, Ogden RT, Mann JJ . Higher 5-HT1A receptor binding potential during a major depressive episode predicts poor treatment response: preliminary data from a naturalistic study. Neuropsychopharmacology 2006; 31: 1745–1749.
Parsey RV, Oquendo MA, Ogden RT, Olvet DM, Simpson N, Huang YY et al. Altered serotonin 1A binding in major depression: a [carbonyl-C-11]WAY100635 positron emission tomography study. Biol Psychiatry 2006; 59: 106–113.
Kaufman J, Sullivan GM, Yang J, Ogden RT, Miller JM, Oquendo MA et al. Quantification of the serotonin 1A receptor using PET: identification of a potential biomarker of major depression in males. Neuropsychopharmacology 2015; 40: 1692–1699.
Miller JM, Hesselgrave N, Ogden RT, Zanderigo F, Oquendo MA, Mann JJ et al. Brain serotonin 1A receptor binding as a predictor of treatment outcome in major depressive disorder. Biol Psychiatry 2013; 74: 760–767.
Iniesta R, Stahl D, McGuffin P . Machine learning, statistical learning and the future of biological research in psychiatry. Psychol Med 2016; 46: 2455–2465.
Chen CC, Schwender H, Keith J, Nunkesser R, Mengersen K, Macrossan P . Methods for identifying SNP interactions: a review on variations of Logic Regression, Random Forest and Bayesian logistic regression. IEEE/ACM Trans Comput Biol Bioinform 2011; 8: 1580–1591.
Baldinger P, Hahn A, Mitterhauser M, Kranz GS, Friedl M, Wadsak W et al. Impact of COMT genotype on serotonin-1A receptor binding investigated with PET. Brain Struct Funct 2014; 219: 2017–2028.
Baldinger P, Hoflich AS, Mitterhauser M, Hahn A, Rami-Mark C, Spies M et al. Effects of Silexan on the serotonin-1A receptor and microstructure of the human brain: a randomized, placebo-controlled, double-blind, cross-over study with molecular and structural neuroimaging. Int J Neuropsychopharmacol 2015; 18: pyu063.
Hahn A, Haeusler D, Kraus C, Hoflich AS, Kranz GS, Baldinger P et al. Attenuated serotonin transporter association between dorsal raphe and ventral striatum in major depression. Hum Brain Mapp 2014; 35: 3857–3866.
Lanzenberger R, Kranz GS, Haeusler D, Akimova E, Savli M, Hahn A et al. Prediction of SSRI treatment response in major depression based on serotonin transporter interplay between median raphe nucleus and projection areas. Neuroimage 2012; 63: 874–881.
Lanzenberger R, Wadsak W, Spindelegger C, Mitterhauser M, Akimova E, Mien LK et al. Cortisol plasma levels in social anxiety disorder patients correlate with serotonin-1A receptor binding in limbic brain regions. Int J Neuropsychopharmacol 2010; 13: 1129–1143.
Kraus C, Baldinger P, Rami-Mark C, Gryglewski G, Kranz GS, Haeusler D et al. Exploring the impact of BDNF Val66Met genotype on serotonin transporter and serotonin-1A receptor binding. PLoS ONE 2014; 9: e106810.
Oeth P, del Mistro G, Marnellos G, Shi T, van den Boom D . Qualitative and quantitative genotyping using single base primer extension coupled with matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MassARRAY). Methods Mol Biol 2009; 578: 307–343.
Wadsak W, Mien LK, Ettlinger DE, Lanzenberger R, Haeusler D . Simple and fully automated preparation of [carbonyl-11C]WAY-100635. Radiochim Acta 2009; 95: 6.
Vanicek T, Spies M, Rami-Mark C, Savli M, Hoflich A, Kranz GS et al. The norepinephrine transporter in attention-deficit/hyperactivity disorder investigated with positron emission tomography. JAMA Psychiatry 2014; 71: 1340–1349.
Sigurdardottir HL, Kranz GS, Rami-Mark C, James GM, Vanicek T, Gryglewski G et al. Effects of norepinephrine transporter gene variants on NET binding in ADHD and healthy controls investigated by PET. Hum Brain Mapp 2014; 37: 884–895.
Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab 2007; 27: 1533–1539.
Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 2002; 15: 273–289.
Pinheiro JC, Bates DM . Mixed-Effects Models in S and S-PLUS. Springer: NewYork, NY, USA, 2000.
Liaw A, Wiener M . Classification and regression by randomForest. R News 2002; 2: 18–22.
Bennett KP, Campbell C . Support vector machines: Hype or hallelujah? SIGKDD Explor 2000; 2. 1–13.
Sullivan GM, Ogden RT, Oquendo MA, Kumar JS, Simpson N, Huang YY et al. Positron emission tomography quantification of serotonin-1A receptor binding in medication-free bipolar depression. Biol Psychiatry 2009; 66: 223–230.
Hirvonen J, Karlsson H, Kajander J, Lepola A, Markkula J, Rasi-Hakala H et al. Decreased brain serotonin 5-HT1A receptor availability in medication-naive patients with major depressive disorder: an in-vivo imaging study using PET and [carbonyl-11C]WAY-100635. Int J Neuropsychopharmacol 2008; 11: 465–476.
Drevets WC, Frank E, Price JC, Kupfer DJ, Holt D, Greer PJ et al. PET imaging of serotonin 1A receptor binding in depression. Biol Psychiatry 1999; 46: 1375–1387.
Sargent PA, Kjaer KH, Bench CJ, Rabiner EA, Messa C, Meyer J et al. Brain serotonin1A receptor binding measured by positron emission tomography with [11C]WAY-100635: effects of depression and antidepressant treatment. Arch Gen Psychiatry 2000; 57: 174–180.
Szewczyk B, Kotarska K, Daigle M, Misztak P, Sowa-Kucma M, Rafalo A et al. Stress-induced alterations in 5-HT1A receptor transcriptional modulators NUDR and Freud-1. Int J Neuropsychopharmacol 2014; 17: 1763–1775.
Malki K, Koritskaya E, Harris F, Bryson K, Herbster M, Tosto MG . Epigenetic differences in monozygotic twins discordant for major depressive disorder. Transl Psychiatry 2016; 6: e839.
Numata S, Ishii K, Tajima A, Iga J, Kinoshita M, Watanabe S et al. Blood diagnostic biomarkers for major depressive disorder using multiplex DNA methylation profiles: discovery and validation. Epigenetics 2015; 10: 135–141.
Le Francois B, Soo J, Millar AM, Daigle M, Le Guisquet AM, Leman S et al. Chronic mild stress and antidepressant treatment alter 5-HT1A receptor expression by modifying DNA methylation of a conserved Sp4 site. Neurobiol Dis 2015; 82: 332–341.
Lolak S, Suwannarat P, Lipsky RH . Epigenetics of depression. Prog Mol Biol Transl Sci 2014; 128: 103–137.
Lockwood LE, Su S, Youssef NA . The role of epigenetics in depression and suicide: A platform for gene-environment interactions. Psychiatry Res 2015; 228: 235–242.
Fuxe K, Borroto-Escuela D, Fisone G, Agnati LF, Tanganelli S . Understanding the role of heteroreceptor complexes in the central nervous system. Curr Protein Pept Sci 2014; 15: 647.
Borroto-Escuela DO, Narvaez M, Perez-Alea M, Tarakanov AO, Jimenez-Beristain A, Mudo G et al. Evidence for the existence of FGFR1-5-HT1A heteroreceptor complexes in the midbrain raphe 5-HT system. Biochem Biophys Res Commun 2015; 456: 489–493.
Borroto-Escuela DO, Perez-Alea M, Narvaez M, Tarakanov AO, Mudo G, Jimenez-Beristain A et al. Enhancement of the FGFR1 signaling in the FGFR1-5-HT1A heteroreceptor complex in midbrain raphe 5-HT neuron systems. Relevance for neuroplasticity and depression. Biochem Biophys Res Commun 2015; 463: 180–186.
Sander CY, Hooker JM, Catana C, Normandin MD, Alpert NM, Knudsen GM et al. Neurovascular coupling to D2/D3 dopamine receptor occupancy using simultaneous PET/functional MRI. Proc Natl Acad Sci USA 2013; 110: 11169–11174.
Mandeville JB, Sander CY, Jenkins BG, Hooker JM, Catana C, Vanduffel W et al. A receptor-based model for dopamine-induced fMRI signal. Neuroimage 2013; 75: 46–57.
Albert PR, Vahid-Ansari F, Luckhart C . Serotonin-prefrontal cortical circuitry in anxiety and depression phenotypes: pivotal role of pre- and post-synaptic 5-HT1A receptor expression. Front Behav Neurosci 2014; 8: 199.
Molina E, Cervilla J, Rivera M, Torres F, Bellon JA, Moreno B et al. Polymorphic variation at the serotonin 1-A receptor gene is associated with comorbid depression and generalized anxiety. Psychiatr Genet 2011; 21: 195–201.
Botta V, Louppe G, Geurts P, Wehenkel L . Exploiting SNP correlations within random forest for genome-wide association studies. PLoS ONE 2014; 9: e93379.
Acknowledgements
We are grateful to the technical and medical teams, especially to K Kletter, R Dudczak, L-K Mien, J Ungersboeck, C Rami-Mark and D Haeusler, of the PET Centre, Medical University of Vienna. Furthermore, we thank A Höflich, C Spindelegger, U Moser, M Fink, D Winkler, R Frey and P Stein for the medical support; and M Savli and A Hahn for the technical support. This research was conducted by pooling data from studies supported by grants of the Oesterreichische Nationalbank (Anniversary Fund, project numbers: 11468, 12809) and the Austrian Science Fund (FWF P16549) to RL and SK, respectively, and an intramural grant of the Department of Psychiatry and Psychotherapy (Forschungskostenstelle). GG is the recipient of a DOC-fellowship of the Austrian Academy of Sciences (OeAW) at the Department of Psychiatry and Psychotherapy. The funders had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
SK received grants/research support, consulting fees and/or honoraria within the last 3 years from Angelini, AOP Orphan Pharmaceuticals AG, AstraZeneca, Eli Lilly, Janssen, KRKA-Pharma, Lundbeck, Neuraxpharm, Pfizer, Pierre Fabre, Schwabe and Servier. RL received travel grants and/or conference speaker honoraria from AstraZeneca, Lundbeck A/S, Dr Willmar Schwabe GmbH, AOP Orphan Pharmaceuticals AG, Janssen-Cilag Pharma GmbH and Roche Austria GmbH. Without any relevance to this work, WW received speaker honoraria from GE Healthcare, research grants from DSD, BSM Diagnostica and ABX and is a part-time employee of CBmed GmbH (Graz, Austria). Without any relevance to this work, MM received speaker honoraria from GE Healthcare. PB-M received a travel grant from AOP Orphan Pharmaceuticals AG and speaker honoraria from Janssen. CK has received travel grants from Roche Austria GmbH and AOP Orphan. GSK received travel grants from Roche and Pfizer. The remaining authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Translational Psychiatry website
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/
About this article
Cite this article
Kautzky, A., James, G., Philippe, C. et al. The influence of the rs6295 gene polymorphism on serotonin-1A receptor distribution investigated with PET in patients with major depression applying machine learning. Transl Psychiatry 7, e1150 (2017). https://doi.org/10.1038/tp.2017.108
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/tp.2017.108
This article is cited by
-
A Comprehensive Review on Major Depressive Disorder: Exploring Etiology, Pathogenesis and Clinical Approaches
Current Behavioral Neuroscience Reports (2025)
-
Major depressive disorder: hypothesis, mechanism, prevention and treatment
Signal Transduction and Targeted Therapy (2024)
-
Genetic contributions to brain serotonin transporter levels in healthy adults
Scientific Reports (2023)
-
Serotonergic gene-to-gene interaction is associated with mood and GABA concentrations but not with pain-related cerebral processing in fibromyalgia subjects and healthy controls
Molecular Brain (2021)
-
Epistasis of HTR1A and BDNF risk genes alters cortical 5-HT1A receptor binding: PET results link genotype to molecular phenotype in depression
Translational Psychiatry (2019)