Abstract
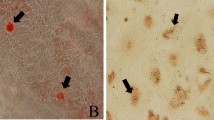
Mammary cell apoptosis and proliferation were assessed after injection of Escherichia coli into the left mammary quarters of six cows. Bacteriological analysis of foremilk samples revealed coliform infection in the injected quarters of four cows. Milk somatic cell counts increased in these quarters and peaked at 24 h after bacterial injection. Body temperature also increased, peaking at 12 h postinjection. The number of apoptotic cells was significantly higher in the mastitic tissue than in the uninfected control. Expression of Bax and interleukin-1β converting enzyme increased in the mastitic tissue at 24 h and 72 h postinfection, whereas Bcl-2 expression decreased at 24 h but did not differ significantly from the control at 72 h postinfection. Induction of matrix metalloproteinase-9, stromelysin-1 and urokinase-type plasminogen activator was also observed in the mastitic tissue. Moreover, cell proliferation increased in the infected tissue. These results demonstrate that Escherichia coli-induced mastitis promotes apoptosis and cell proliferation. Cell Death and Differentiation (2001) 8, 808–816
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- CFU:
-
colony forming units
- E. coli :
-
Escherichia coli
- ECM:
-
extracellular matrix, ICE, interleukin-1β converting enzyme
- IL-1:
-
interleukin-1
- LPS:
-
lipopolysaccharide
- MMP:
-
matrix metalloproteinase
- PA:
-
plasminogen activator
- PAI:
-
plasminogen activator inhibitor
- RT-PCR:
-
reverse-transcriptase polymerase chain reaction
- S. agalactiae :
-
Streptococcus agalactiae
- S. aureus :
-
Staphylococcus aureus
- SCC:
-
somatic cell counts
- SL:
-
stromelysin
- SV40 Tag:
-
Simian virus 40 T antigen
- TIMP:
-
tissue inhibitor of metalloproteinase
- TNF α:
-
tumor necrosis factor α
References
Blowey R, Edmondson P . 1995 Mastitis-causes, epidemiology and control. In: Blowey R and Edmondson P (eds) Mastitis control in dairy herds: an illustrated and practical guide Farming Press: Ipswich, UK pp 27–45
Eberhart RJ . 1977 Coliform mastitis J.A.V.M.A. 170: 1160–1163
Eberhart RJ, Natzke RP, Newbould FHS, Nonnecke B, Thompson P . 1979 Coliform mastitis – a review J. Dairy Sci. 62: 1–22
Hill AW . 1994 Escherichia coli mastitis. In: Gyles CL (ed) Escherichia coli in domestic animals and humans Cab International: Wallingford, UK pp 117–133
Hogan JS, Smith KL, Hoblet KH . 1989 Field survey of clinical mastitis in low somatic cell count herds J Dairy Sci 72: 1547–1556
Thompson CB . 1995 Apoptosis in the pathogenesis and treatment of disease Science 267: 1445–1449
Sheffield LG . 1997 Mastitis increases growth factor messenger ribonucleic acid in bovine mammary glands J. Dairy Sci. 80: 2020–2024
Bayles KW, Wesson CA, Liou LE, Fox LK, Bohach GA, Trumble WR . 1998 Intracellular Staphylococcus aureus escapes the endosome and induces apoptosis in epithelial cells Infect. Immun. 66: 336–342
Adams JM, Cory S . 1998 The Bcl-2 protein family: Arbiters of cell survival Science 281: 1322–1326
Thornberry NA, Lazebnik Y . 1998 Caspases: Enemies within Science 281: 1312–1316
Boudreau N, Sympson CJ, Werb Z, Bissell MJ . 1995 Suppression of ICE and apoptosis in mammary epithelial cells by extracellular matrix Science 267: 891–893
Pullan S, Wilson J, Metcalfe A, Edwards GM, Goberdhan N, Tilly J, Hickman JA, Dive C, Streui CH . 1996 Requirement of basement membrane for the suppression of programmed cell death in mammary epithelium J. Cell Sci. 109: 631–642
Merlo GR, Cella N, Hynes NE . 1997 Apoptosis is accompanied by changes in Bcl-2 and Bax expression, induced by loss of attachment, and inhibited by specific extracellular matrix proteins in mammary epithelial cells Cell Growth Differ. 8: 251–260
Lund LR, Romer J, Thomasset N, Solberg H, Pyke C, Bissell MJ, Dane K, Werb Z . 1996 Two distinct phases of apoptosis in mammary gland involution: proteinase-independent and -dependent pathways Development 122: 181–193
Strange R, Li F, Saurer S, Burkhardt A, Friis RR . 1992 Apoptotic cell death and tissue remodelling during mouse mammary gland involution Development 115: 49–58
Alexander CM, Howard EW, Bissell MJ, Werb Z . 1996 Rescue of mammary epithelial cell apoptosis and entactin degradation by a tissue inhibitor of metallproteinase-1 transgene J. Cell Biol. 135: 1669–1677
Matthews KR, Rejman JJ, Turner JD, Oliver SP . 1994 Proliferation of a bovine mammary epithelial cell line in the presence of bacterial virulence factors J. Dairy Sci. 77: 2959–2964
Heermeier H, Benedict M, Li M, Furth P, Nuñez G, Hennighausen L . 1996 Bax and Bcl-xS are induced at the onset of apoptosis in involuting mammary epithelial cells Mech. Dev. 56: 197–207
Metcalfe AD, Gilmore A, Klinowska T, Oliver J, Valentijn AJ, Brown R, Ross A, MacGregor G, Hickman JA, Streuli CH . 1999 Developmental regulation of Bcl-2 family protein expression in the involuting mammary gland J. Cell Sci. 112: 1771–1783
Schorr K, Li M, Krajewski S, Reed JC, Furth PA . 1999 Bcl-2 gene family and related proteins in mammary gland involution and breast cancer J. Mam. Gland Biol. Neoplasia 4: 153–164
Li M, Hu J, Heermeier K, Hennighausen L, Furth PA . 1996 Expression of a viral oncoprotin during mammary gland development alters cell fate and function: induction of p53-independent apoptosis is followed by impaired milk protein production in surviving cells Cell Growth Differ. 7: 3–11
Tzeng YJ, Gottlob K, Santarelli R, Graessmann A . 1996 The SV40 T-antigen induces premature apoptotic mammary gland involution during late pregnancy in transgenic mice FEBS Lett. 380: 215–218
Schorr K, Li M, Bar-Peled U, Lewis A, Heredia A, Lewis B, Knudson M, Korsmeyer SJ, Jäger R. Weiher H, Furth PA . 1999 Gain of Bcl-2 is more potent than Bax Loss in regulating mammary epithelial cell survival in vivo Cancer Res. 592: 541–2545
Keane MM, Ettenberg SA, Lowrey GA, Russell EK, Lipkowitz S . 1996 Fas expression and function in normal and malignant breast cell lines Cancer Res. 56: 4791–4798
Maloof P, Wang Q, Wang H, Stein D, Denny TN, Yahalom J, Fenig E, Wieder R . 1999 Overexpression of basic fibroblast growth factor (FGF-2) downregulates Bcl-2 and promotes apoptosis in MCF-7 human breast cancer cells Breast Cancer Res. Treat. 56: 153–167
Colitti M, Stefanon B, Wilde CJ . 1999 Apoptotic cell death, bax and bcl-2 expression during sheep mammary gland involution Anat. Histol. Embryol. 28: 257–264
Hazan RB, Phillips GR, Qiao RF, Norton L, Aaronson SA . 2000 Exogenous expression of N-cadherin in breast cancer cells induces cell migration, invasion and Metastasis J. Cell Biol. 148: 779–790
Legrand C, Gilles C, Zahm J-M, Polette M, Buisson A-C, Kaplan H, Birembaut P, Tournier J-M . 1999 Airway epithelial cell migration dynamics: MMP-9 role in cell-extracellular matrix remodeling J. Cell Biol. 146: 517–529
Li X, Zhao X, Ma S . 1999 Secretion of 92 kDa gelatinase (MMP-9) by bovine neutrophils Vet. Immunol. Immunopathol. 67: 247–258
Reddy KB, Krueger JS, Kondapaka SB, Diglio CA . 1999 Mitogen-activated protein kinase (MAPK) regulates the expression of progelatinase B (MMP-9) in breast epithelial cells Int. J. Cancer 82: 268–273
Shuster DE, Lee EK, Kehrli Jr ME . 1996 Bacterial growth, inflammatory cytokine production, and neutrophil recruitment during coliform mastitis in cows within ten days after calving, compared with cows at midlactation Am. J. Vet. Res. 57: 1569–1575
Roh CR, Oh WJ, Yoon BK, Lee JH . 2000 Up-regulation of matrix metalloproteinase-9 in human myometrium during labour: a cytokine-mediated process in uterine smooth muscle cells Mol. Hum. Reprod. 6: 96–102
Rudolph-Owen LA, Matrisian LM . 1998 Matrix metalloproteinases in remodeling of the normal and neoplastic mammary gland J. Mam. Gland Biol. Neoplasia 3: 177–189
Keller NR, Sierra-Rivera E, Eisenberg E, Osteen KG . 2000 Progesterone exposure prevents matrix metalloproteinase-3 (MMP-3) stimulation by interleukin-1 alpha in human endometrial stromal cells J. Clin. Endocrinol. Metab. 85: 1611–1619
Siwik DA, Chang DL, Colucci WS . 2000 Interleukin-1 beta and tumor necrosis factor-alpha decrease collagen synthesis and increase matrix metalloproteinase activity in cardiac fibroblasts in vitro Circul. Res. 86: 1259–1265
Zavizion B, White JH, Bramley AJ . 1997 Staphylococcus aureus stimulates urokinase-type plasminogen activator expression by bovine mammary cells J. Infect. Dis. 76: 1637–1640
Ohta S, Niiya K, Sakuragawa N, Fuse H . 2000 Induction of urokinase-type plasminogen activator by lipopolysaccharide in PC-3 human prostatic cancer cells Thrombosis Res. 97: 343–347
Swank GM, Lu Q, Xu D-Z, Michalsky M, Deitch EA . 1998 Effect of acute-phase and heat-shock stress on apoptosis in intestinal epithelial cells (Caco-2) Crit. Care Med. 26: 1213–1217
Mebmer UK, Briner VA, Pfeilschifter J . 1999 Tumor necrosis factor-α and lipopolysaccharide induce apoptotic cell death in bovine glomerular endothelial cells Kidney Int. 55: 2322–2337
Capuco AV, Paape MJ, Smith JJ, Loefler DA . 1985 In vitro effect of bacterial toxins on lactating bovine mammary tissue J. Dairy Sci. 68 Suppl 1: 206–207
Boudjellab N, Chan-Tang HS, Zhao X . 2000 Bovine interleukin-1 expression by cultured mammary epithelial cells (MAC-T) and its involvement in the release of MAC-T derived interleukin-8 Comp. Physiol. Biochem. 127: 191–199
Shuster DE, Kehrli Jr ME, Rainard P, Paape M . 1997 Complement fragment C5a and inflammatory cytokines in neutrophil recruitment during intramammary infection with Escherichia coli Infect. Immun. 65: 3286–3292
Burow ME, Tang Y, Collins-Burow BM, Krajewski S, Reed JC, McLachlan JA, Bechman BS . 1999 Effects of environmental estrogens on tumor necrosis factor α-mediated apoptosis in MCF-7 cells Carcinogenesis 20: 2057–2061
Kampf C, Relova AJ, Sandler S, Roomans GM . 1999 Effects of TNF-alpha, IFN-gamma and IL-1beta on normal human bronchial epithelial cells Eur. Respir. J. 14: 84–91
Tanaka T, Umesaki N, Mizuno K, Chang L, Ohtaki S, Ogita S . 1998 Enhancement of apoptotic susceptibility by interleukin-1 beta in human endometrial epithelial cells Gynecol. Endocrinol. 12: 315–319
Furth PA, Bar-Peled U, Li M, Lewis A, Laucirica R, Jäger R, Weiher H, Russell RG . 1999 Loss of anti-mitotic effects of Bcl-2 with retention of anti-apoptotic activity during tumor progression in a mouse model Oncogene 18: 6589–6596
Capuco AV, Akers RM . 1990 Thymidine incorporation by lactating mammary epithelium during compensatory mammary growth in beef cattle J. Dairy Sci. 73: 3094–3103
Wesson CA, Deringer J, Liou LE, Bayles KW, Bohach GA, Trumble WR . 2000 Apoptosis induced by Staphylococcus aureus in epithelial cells utilizes a mechanism involving caspases 8 and 3 Infect. Immun. 68: 2998–3001
Farr VC, Stelwagen K, Cate LR, Molenaar AJ, McFadden TB, Davis SR . 1996 An improved method for the routine biopsy of bovine mammary tissue J. Dairy Sci. 79: 543–549
Bramley AJ . 1976 Variations in the susceptibility of lactating and non-lactating bovine udders to infection when infused with Escherichia coli J. Dairy Res. 43: 205–211
Hill AW . 1981 Factors influencing the outcome of Escherichia coli mastitis in the dairy cow Res. Vet. Sci. 31: 107–112
Li M, Hu J, Heermeier K, Hennighausen L, Furth PA . 1996 Apoptosis and remodeling of mammary gland tissue during involution proceeds through p53-independent pathways Cell Growth Differ. 7: 13–20
Rueda BR, Tilly KI, Botros IW, Jolly PD, Hansen TR, Hoyer PB, Tilly JL . 1997 Increased bax and interleukin-1β-converting enzyme messenger ribonucleic acid levels coincide with apoptosis in the bovine corpus luteum during structural regression Biol. Reprod. 56: 186–193
Acknowledgements
This work was partially supported by a grant (155423-98) from Natural Sciences and Engineering Research Council of Canada to X Zhao.
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by G Nunez
Rights and permissions
About this article
Cite this article
Long, E., Capuco, A., Wood, D. et al. Escherichia coli induces apoptosis and proliferation of mammary cells. Cell Death Differ 8, 808–816 (2001). https://doi.org/10.1038/sj.cdd.4400878
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/sj.cdd.4400878
Keywords
This article is cited by
-
Lactobacillus plantarum 17–5 attenuates Escherichia coli-induced inflammatory responses via inhibiting the activation of the NF-κB and MAPK signalling pathways in bovine mammary epithelial cells
BMC Veterinary Research (2022)
-
Metagenomic deep sequencing reveals association of microbiome signature with functional biases in bovine mastitis
Scientific Reports (2019)
-
Cell free mitochondrial DNA in serum and milk associated with bovine mastitis: a pilot study
Veterinary Research Communications (2018)
-
Lactobacillus rhamnosus GR-1 Limits Escherichia coli-Induced Inflammatory Responses via Attenuating MyD88-Dependent and MyD88-Independent Pathway Activation in Bovine Endometrial Epithelial Cells
Inflammation (2016)
-
Gene expression profiling of porcine mammary epithelial cells after challenge with Escherichia coli and Staphylococcus aureus in vitro
Veterinary Research (2015)