Abstract
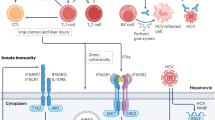
Cellular immune responses play an important role in the control of hepatitis C virus (HCV), although in the majority of cases they ultimately fail. We examine the mechanisms by which virus-specific T cells may interact with a cell that is infected with HCV and how this interaction may explain the success and failure of the immune response. As an infected cell presenting foreign antigen, the hepatocyte will interact with a large number of lymphocytes, both by direct cell to cell contact and by indirect means through the secretion of cytokines and chemokines. These interactions may lead on the one hand to the death of infected hepatocytes or suppression of viral replication and on the other hand to the death of T lymphocytes or down regulation of their function. We suggest that activation of lymphocytes in lymphoid organs leads to generation of effector T cells (positive loop), while at the same time presentation of antigen in the liver either on hepatocytes or other specialised antigen presenting cells depresses these responses (negative loop). This model helps to explain both the specific phenotype and low frequencies of HCV specific CTL in chronic infection, through early elimination of cells before expansion and maturation can occur. The outcome of HCV infection is likely to result from the early balance between these two simultaneous loops.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- APC:
-
antigen presenting cell
- CMV:
-
cytomegalovirus
- CTL:
-
cytotoxic T lymphocytes
- EBV:
-
Epstein–Barr virus
- HBV:
-
hepatitis B virus
- HCV:
-
hepatitis C virus
- IFN:
-
interferon
- IL:
-
interleukin
- IP-10:
-
IFN-γ induced protein 10
- LCMV:
-
lymphocytic choriomeningitis virus
- LSEC:
-
liver sinusoidal epithelial cells
- MHC:
-
major histocompatibility complex
- MIG:
-
monokine induced by IFN-γ
- PBMC:
-
peripheral blood mononuclear cells
- PCR:
-
polymerase chain reaction
References
Cohen J (1999) The scientific challenge of hepatitis C. Science 285: 26–30.
Frank C, Mohamed MK, Strickland GT, Lavanchy D, Arthur RR, Magder LS, El Khoby T, Abdel-Wahab Y, Aly Ohn ES, Anwar W & Sallam I (2000) The role of parenteral antischistosomal therapy in the spread of hepatitis C virus in Egypt. Lancet 355: 887–891.
Lauer GM & Walker BD (2001) Hepatitis C virus infection. N. Engl. J. Med. 345: 41–52.
Klenerman P, Lucas M, Barnes E & Harcourt G (2002) Immunity to hepatitis C virus: stunned but not defeated. Microbes Infect. 4: 57–65.
VanderBroek M, Muller U, Hang S, Aguet M & Zinkernagel R (1995) Antiviral immunity in mice lacking both alpha/beta and gamma interferon receptors. J. Virol. 69: 4792–4796.
Karrer U, Althage A, Odermatt B, Hengartner H & Zinkernagel RM (2000) Immunodeficiency of alymphoplasia mice (aly/aly) in vivo: structural defect of secondary lymphoid organs and functional B cell defect. Eur. J. Immunol. 30: 2799–2807.
Planz O, Ehl S, Furrer E, Horvath E, Brundler M, Hengartner H & Zinkernagel R (1997) A critical role for neutralising antibody-producing B cells, CD4+ cells and interferons in persistent infections of mice with LCMV: implications for adoptive immunotherapy of virus carriers. Proc. Natl. Acad. Sci USA 94: 6874–6879.
Ciurea A, Klenerman P, Hunziker L, Horvath E, Senn BM, Ochsenbein AF, Hengartner H & Zinkernagel RM (2000) Viral persistence in vivo through selection of neutralizing antibody-escape variants. Proc. Natl. Acad. Sci. USA 97: 2749–2754.
Zajac AJ, Blattman JN, Murali-Krishna K, Sourdive DJ, Suresh M, Altman JD & Ahmed R (1998) Viral immune evasion due to persistence of activated T cells without effector function. J. Exp. Med. 188: 2205–2213.
Zajac A, Blattman J, Murali-Krishna K, Sourdive D, Suresh M, Altman J & Ahmed R (1998) Viral immune evasion due to persistence of activated T cells without effector function. J. Exp. Med. 188: 2205–2213.
Klenerman P, Cerundolo V & Dunbar R (2002) Tracking T cells with tetramers: New Tales from new tools. Nature Rev. 2: 263–271.
Lechner F, Wong DK, Dunbar PR, Chapman R, Chung RT, Dohrenwend P, Robbins G, Phillips R, Klenerman P & Walker BD (2000) Analysis of successful immune responses in persons infected with hepatitis C virus. J. Exp. Med. 191: 1499–1512.
Lechner F, Gruener N, Urbani S, Uggeri J, Santantonio T, Cerny A, Phillips R, Ferrari C, Pape G & Klenerman P (2000) CTL responses are induced during acute HCV infection but are not sustained. Eur. J. Immunol. 30: 2479–2487.
Thimme R, Oldach D, Chang KM, Steiger C, Ray SC & Chisari FV (2001) Determinants of viral clearance and persistence during acute hepatitis C virus infection. J. Exp. Med. 194: 1395–1406.
Cooper S, Erickson A, Adams E, Kansopon J, Weiner A, Chien D, Houghton M, Parham P & Walker C (1999) Analysis of a successful immune response against HCV. Immunity 10: 439–449.
Thursz M, Yallop R, Goldin R, Trepo C & Thomas HC (1999) Influence of MHC class II genotype on outcome of infection with hepatitis C virus. The HENCORE group. Hepatitis C European Network for Cooperative Research. Lancet 354: 2119–2124.
Harcourt G, Hellier S, Bunce M, Satsangi J, Collier J, Chapman R, Phillips R & Klenerman P (2001) Effect of HLA Class II genptype on T helper lymphocyte responses and viral control in HCV infection. J. Viral. Hepat. 8: 174–179.
Gerlach J, Diepolder H, Jung M-C, Gruener N, Schraut W, Zachoval R, Hoffman R, Schirren C, Santantonio T & Pape G (1999) Recurrence of HCV after loss of virus specific CD4+ T cell response in acute Hepatitis C. Gastroenterology 117: 933–941.
He X-S, Rehermann B, Lopez-Labrador F, Boisvert J, Cheung R, Mumm J, Wedermeyer H, Berenguer M, Wright T & Davis M (1999) Quantitative analysis of HCV-specific CD8+ T cells in peripheral blood and liver using peptide-MHC tetramers. Proc. Natl. Acad. Sci (USA) 96: 5692–5697.
Gruener N, Lechner F, Jung M-C, Diepolder H, Gerlach T, Lauer G, Walker B, Sullivan J, Phillips R & Pape G (2001) Sustained dysfunction of antiviral CD8+ T lymphocytes after infection with HCV. J. Virol. 75: 5550–5558.
Lauer GM, Nguyen TN, Day CL, Robbins GK, Flynn T, McGowan K, Rosenberg ES, Lucas M, Klenerman P, Chung RT & Walker BD (2002) Human immunodeficiency virus type 1-hepatitis C virus coinfection: intraindividual comparison of cellular immune responses against two persistent viruses. J. Virol. 76: 2817–2826.
Lauer G, Ouchi K, Chung R, Nguyen T, Day C, Purkis D, Reiser M, Kim AY, Lucas M, Klenerman P & Walker BD (2002) Comprehensive analysis of CD8+ T-cell responses against HCV reveals multiple unpredicted specificities. J. Virol. 76: 6104–6113.
Wong DK, Dudley DD, Afdhal NH, Dienstag J, Rice CM, Wang L, Houghton M, Walker BD & Koziel MJ (1998) Liver-derived CTL in hepatitis C virus infection: breadth and specificity of responses in a cohort of persons with chronic infection. J. Immunol. 160: 1479–1488.
Cramp ME, Rossol S, Chokshi S, Carucci P, Williams R & Naoumov NV (2000) Hepatitis C virus-specific T-cell reactivity during interferon and ribavirin treatment in chronic hepatitis C. Gastroenterology 118: 346–355.
Grabowska AM, Lechner F, Klenerman P, Tighe PJ, Ryder S, Ball JK, Thomson BJ, Irving WL & Robins RA (2001) Direct ex vivo comparison of the breadth and specificity of the T cells in the liver and peripheral blood of patients with chronic HCV infection. Eur. J. Immunol. 31: 2388–2394.
Scotet E, Peyrat MA, Saulquin X, Retiere C, Couedel C, Davodeau F, Dulphy N, Toubert A, Bignon JD, Lim A, Vie H, Hallet MM, Liblau R, Weber M, Berthelot JM, Houssaint E & Bonneville M (1999) Frequent enrichment for CD8 T cells reactive against common herpes viruses in chronic inflammatory lesions: towards a reassessment of the physiopathological significance of T cell clonal expansions found in autoimmune inflammatory processes. Eur. J. Immunol. 29: 973–985.
Weiner D, Erickson A, Kansopon J, Crawford K, Muchmore E, Hughes A, Houghton M & Walker C (1995) Persistent Hepatitis C infection in a chimpanze is associated with emergence of a CTL escape variant. Proc. Nat. Acad. Sci. USA 82: 2755–2759.
Chang K, Rehermann B, McHutchinson J, Pasquellini C, Southwood S, Sette A & Chisari F (1997) Immunological significance of CTL epitope variants in patients chronically infected by HCV. J. Clin. Invest. 100: 2376–2385.
Farci P, Shimoda A, Coiana A, Diaz G, Peddis G, Melpolder JC, Strazzera A, Chien DY, Munoz SJ, Balestrieri A, Purcell RH & Alter HJ (2000) The outcome of acute hepatitis C predicted by the evolution of the viral quasispecies. Science 288: 339–344.
Moskophidis D, Lechner F, Pircher H & Zinkernagel RM (1993) Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature 362: 758–761.
Gallimore A, Glitheroe A, Godkin A, Tissot A, Hengartner H, Elliott T & Zinkernagel R (1998) Induction and exhaustion of LCMV-specific CTL visualised using soluble tetrameric MHC-peptide complexes. J. Exp. Med. 187: 1383–1393.
Battegay M, Moskophidis D, Rahemtulla A, Hengartner H, Mak TW & Zinkernagel RM (1994) Enhanced establishment of a virus carrier state in adult CD4+ T-cell-deficient mice. J. Virol. 68: 4700–4704.
Ou R, Zhou S, Huang L & Moskophidis D (2001) Critical role for alpha/beta and gamma interferons in persistence of lymphocytic choriomeningitis virus by clonal exhaustion of cytotoxic T cells. J. Virol. 75: 8407–8423.
Large MK, Kittlesen DJ & Hahn YS (1999) Suppression of host immune response by the core protein of hepatitis C virus: possible implications for hepatitis C virus persistence. J. Immunol. 162: 931–938.
Appay V, Nixon DF, Donahoe SM, Gillespie GM, Dong T, King A, Ogg GS, Spiegel HM, Conlon C, Spina CA, Havlir DV, Richman DD, Waters A, Easterbrook P, McMichael AJ & Rowland-Jones SL (2000) HIV-specific CD8(+) T cells produce antiviral cytokines but are impaired in cytolytic function. J. Exp. Med. 192: 63–75.
Appay V, Dunbar R, Callan M, Klenerman P, Gillespie GMA, Papagno L, Ogg GS, King A, Lechner F, Spina CA, Little S, Havlir DV, Richman DD, Gruener N, Pape G, Waters A, Easterbrook P, Salio M, Cerundolo V, McMichael AJ & Rowland-Jones SL (2002) Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nature Med. 8: 379–385.
Calne RY, Sells RA, Pena JR, Davis DR, Millard PR, Herbertson BM, Binns RM & Davies DA (1969) Induction of immunological tolerance by porcine liver allografts. Nature 223: 472–476.
Bertolino P, Trescol-Biemont MC & Rabourdin-Combe C (1998) Hepatocytes induce functional activation of naive CD8+ T lymphocytes but fail to promote survival. Eur. J. Immunol. 28: 221–236.
Mehal WZ, Azzaroli F & Crispe IN (2001) Antigen presentation by liver cells controls intrahepatic T cell trapping, whereas bone marrow-derived cells preferentially promote intrahepatic T cell apoptosis. J. Immunol. 167: 667–673.
Knolle PA & Gerken G (2000) Local control of the immune response in the liver. Immunol. Rev. 174: 21–34.
Schroder AJ, Blaheta RA, Scholz M, Kronenberger B, Encke A & Markus BH (1995) Effects of proinflammatory cytokines on cultivated primary human hepatocytes. Fluorometric measurement of intercellular adhesion molecule-1 and human leukocyte antigen-A, -B, -C, and -DR expression. Transplantation 59: 1023–1028.
Beersma MF, Bijlmakers MJ & Ploegh HL (1993) Human cytomegalovirus down-regulates HLA class I expression by reducing the stability of class I H chains. J. Immunol. 151: 4455–4464.
Moradpour D, Grabscheid B, Kammer AR, Schmidtke G, Groettrup M, Blum HE & Cerny A (2001) Expression of hepatitis C virus proteins does not interfere with major histocompatibility complex class I processing and presentation in vitro. Hepatology 33: 1282–1287.
Kafrouni MI, Brown GR & Thiele DL (2001) Virally infected hepatocytes are resistant to perforin-dependent CTL effector mechanisms. J. Immunol. 167: 1566–1574.
Guidotti LG, Rochford R, Chung J, Shapiro M, Purcell R & Chisari FV (1999) Viral clearance without destruction of infected cells during acute HBV infection. Science 284: 825–829.
Kagi D, Seiler P, Pavlovic J, Ledermann B, Burki K, Zinkernagel RM & Hengartner H (1995) The roles of perforin- and Fas-dependent cytotoxicity in protection against cytopathic and noncytopathic viruses. Eur. J. Immunol. 25: 3256–3262.
Fiore G, Piazzolla G, Galetta V, Caccetta L, Schiraldi O & Antonaci S (1999) Liver tissue expression of CD80 and CD95 antigens in chronic hepatitis C: relationship with biological and histological disease activities. Microbioscience 97: 29–38.
Ando K, Hiroishi K, Kaneko T, Moriyama T, Muto Y, Kayagaki N, Yagita H, Okumura K & Imawari M (1997) Perforin, Fas/Fas ligand, and TNF-alpha pathways as specific and bystander killing mechanisms of hepatitis C virus-specific human CTL. J. Immunol. 158: 5283–5291.
Galle PR, Hofmann WJ, Walczak H, Schaller H, Otto G, Stremmel W, Krammer PH & Runkel L (1995) Involvement of the CD95 (APO-1/Fas) receptor and ligand in liver damage. J. Exp. Med. 182: 1223–1230.
Stonans I, Stonane E, Russwurm S, Deigner HP, Bohm KJ, Wiederhold M, Jager L & Reinhart K (1999) HepG2 human hepatoma cells express multiple cytokine genes. Cytokine 11: 151–156.
Shen X, Hong F, Nguyen VA & Gao B (2000) IL-10 attenuates IFN-alpha-activated STAT1 in the liver: involvement of SOCS2 and SOCS3. FEBS Lett. 480: 132–136.
Narumi S, Tominaga Y, Tamaru M, Shimai S, Okumura H, Nishioji K, Itoh Y & Okanoue T (1997) Expression of IFN-inducible protein-10 in chronic hepatitis. J. Immunol. 158: 5536–5544.
Nishioji K, Okanoue T, Itoh Y, Narumi S, Sakamoto M, Nakamura H, Morita A & Kashima K (2001) Increase of chemokine interferon-inducible protein-10 (IP-10) in the serum of patients with autoimmune liver diseases and increase of its mRNA expression in hepatocytes. Clin. Exp. Immunol. 123: 271–279.
Kakimi K, Lane TE, Wieland S, Asensio VC, Campbell IL, Chisari FV & Guidotti LG (2001) Blocking chemokine responsive to gamma-2/interferon (IFN)-gamma inducible protein and monokine induced by IFN-gamma activity in vivo reduces the pathogenetic but not the antiviral potential of hepatitis B virus-specific cytotoxic T lymphocytes. J. Exp. Med. 194: 1755–1766.
Bain C, Fatmi A, Zoulim F, Zarski JP, Trepo C & Inchauspe G (2001) Impaired allostimulatory function of dendritic cells in chronic hepatitis C infection. Gastroenterology 120: 512–524.
Ochsenbein AF, Sierro S, Odermatt B, Pericin M, Karrer U, Hermans J, Hemmi S, Hengartner H & Zinkernagel RM (2001) Roles of tumour localization, second signals and cross priming in cytotoxic T-cell induction. Nature 411: 1058–1064.
Klenerman P, Lechner F, Kantzanou M, Ciurea A, Hengartner H & Zinkernagel R (2000) Viral escape and the failure of cellular immune responses. Science 289: 2003
Ward S, Lauer G, Isba R, Walker B & Klenerman P (2002) Cellular immune responses against hepatitis C virus: the evidence base 2002. Clin. Exp. Immunol. 128: 195–203.
Cerny A, Fowler P, Brothers M, Houghton M, Schlicht H & Chisari F (1995) Induction in vitro of a primary human antiviral cytotoxic T cell response. Eur. J. Immnuol. 25: 627–630.
Rehermann B, Chang K, McHutchinson J, Kokka R, Houghton M & Chisari F (1996) Quantitative analysis of the peripheral blood CTL response in patients with chronic HCV infection. J. Clin. Invest. 98: 1432–1440.
Cruickshank SM, Southgate J, Selby PJ & Trejdosiewicz LK (1998) Expression and cytokine regulation of immune recognition elements by normal human biliary epithelial and established liver cell lines in vitro. J. Hepatol. 29: 550–558.
Wang D, Yang E & Cheng LY (1997) Effects of IFN-gamma, TNF-alpha and EGF on the expression of HLA class I antigen and the proliferation of human hepatocellular carcinoma HepG2 cells. Anticancer Res. 17: 181–188.
Paroli M, Carloni G, Franco A, De Petrillo G, Alfani E & Barnaba V (1993) Human hepatoma cells expressing HLA class I molecules stimulate primary responses of purified CD8+ T lymphocytes. Res. Virol. 144: 327–332.
Paroli M, Carloni G, Franco A, De Petrillo G, Alfani E, Perrone A & Barnaba V (1994) Human hepatoma cells expressing MHC antigens display accessory cell function: dependence on LFA-1/ICAM-1 interaction. Immunology 82: 215–221.
Ren EC, Wong LY & Chan SH (1988) Effect of recombinant gamma interferon on hepatocellular carcinoma cell lines. Cancer Lett. 39: 305–310.
Mickelson JK, Kukielka G, Bravenec JS, Mainolfi E, Rothlein R, Hawkins HK, Kelly JH & Smith CW (1995) Differential expression and release of CD54 induced by cytokines. Hepatology 22: 866–875.
Gulubova MV (1998) Intercellular adhesion molecule-1 (ICAM-1) expression in the liver of patients with extrahepatic cholestasis. Acta Histochem. 100: 59–74.
Desbarats J & Newell MK (2000) Fas engagement accelerates liver regeneration after partial hepatectomy. Nat. Med. 6: 920–923.
Lamboley C, Bringuier AF & Feldmann G (2000) Apoptotic behaviour of hepatic and extra-hepatic tumor cell lines differs after Fas stimulation. Cell. Mol. Biol. (Noisy-le-grand) 46: 13–28.
Roskams T, Libbrecht L, Van Damme B & Desmet V (2000) Fas and Fas ligand: strong co-expression in human hepatocytes surrounding hepatocellular carcinoma; can cancer induce suicide in peritumoural cells?. J. Pathol. 191: 150–153.
Muller M, Strand S, Hug H, Heinemann EM, Walczak H, Hofmann WJ, Stremmel W, Krammer PH & Galle PR (1997) Drug-induced apoptosis in hepatoma cells is mediated by the CD95 (APO-1/Fas) receptor/ligand system and involves activation of wild-type p53. J. Clin. Invest. 99: 403–413.
Afford SC, Randhawa S, Eliopoulos AG, Hubscher SG, Young LS & Adams DH (1999) CD40 activation induces apoptosis in cultured human hepatocytes via induction of cell surface fas ligand expression and amplifies fas-mediated hepatocyte death during allograft rejection. J. Exp. Med. 189: 441–446.
Satoh T, Ichida T, Matsuda Y, Sugiyama M, Yonekura K, Ishikawa T & Asakura H (2000) Interaction between hyaluronan and CD44 in the development of dimethylnitrosamine-induced liver cirrhosis. J. Gastroenterol. Hepatol. 15: 402–411.
Mathew J, Hines JE, Obafunwa JO, Burr AW, Toole K & Burt AD (1996) CD44 is expressed in hepatocellular carcinomas showing vascular invasion. J. Pathol. 179: 74–79.
Kronenberger B, Ruster B, Elez R, Weber S, Piiper A, Lee JH, Roth WK & Zeuzem S (2001) Interferon alfa down-regulates CD81 in patients with chronic hepatitis C. Hepatology 33: 1518–1526.
Trobonjaca Z, Leithauser F, Moller P, Schirmbeck R & Reimann J (2001) Activating immunity in the liver. I. Liver dendritic cells (but not hepatocytes) are potent activators of IFN-gamma release by liver NKT cells. J. Immunol. 167: 1413–1422.
Higuchi H, Bronk SF, Takikawa Y, Werneburg N, Takimoto R, El-Deiry W & Gores GJ (2001) The bile acid glycochenodeoxycholate induces trail-receptor 2/DR5 expression and apoptosis. J. Biol. Chem. 276: 38610–38618.
Mochizuki K, Hayashi N, Katayama K, Hiramatsu N, Kanto T, Mita E, Tatsumi T, Kuzushita N, Kasahara A, Fusamoto H, Yokochi T & Kamada T (1997) B7/BB-1 expression and hepatitis activity in liver tissues of patients with chronic hepatitis C. Hepatology 25: 713–718.
Kano A, Watanabe Y, Takeda N, Aizawa S & Akaike T (1997) Analysis of IFN-gamma-induced cell cycle arrest and cell death in hepatocytes. J. Biochem. (Tokyo) 121: 677–683.
Beyer HS & Stanley M (1990) Tumor necrosis factor-alpha increases hepatic DNA and RNA and hepatocyte mitosis. Biochem. Int. 22: 405–410.
Jones BE, Lo CR, Liu H, Srinivasan A, Streetz K, Valentino KL & Czaja MJ (2000) Hepatocytes sensitized to tumor necrosis factor-alpha cytotoxicity undergo apoptosis through caspase-dependent and caspase-independent pathways. J. Biol. Chem. 275: 705–712.
Kim WH, Hong F, Jaruga B, Hu Z, Fan S, Liang TJ & Gao B (2001) Additive activation of hepatic NF-kappaB by ethanol and hepatitis B protein X (HBX) or HCV core protein: involvement of TNF-alpha receptor 1-independent and -dependent mechanisms. FASEB J. 15: 2551–2553.
Szabo G, Catalano D, Bellerose G & Mandrekar P (2001) Interferon alpha and alcohol augment nuclear regulatory factor-kappaB activation in HepG2 cells, and interferon alpha increases pro-inflammatory cytokine production. Alcohol Clin. Exp. Res. 25: 1188–1197.
Satoh S, Nussler AK, Liu ZZ & Thomson AW (1994) Proinflammatory cytokines and endotoxin stimulate ICAM-1 gene expression and secretion by normal human hepatocytes. Immunology 82: 571–576.
Gabay C, Porter B, Guenette D, Billir B & Arend WP (1999) Interleukin-4 (IL-4) and IL-13 enhance the effect of IL-1beta on production of IL-1 receptor antagonist by human primary hepatocytes and hepatoma HepG2 cells: differential effect on C-reactive protein production. Blood 93: 1299–1307.
Lagadic-Gossmann D, Lerche C, Rissel M, Joannard F, Galisteo M, Guillouzo A & Corcos L (2000) The induction of the human hepatic CYP2E1 gene by interleukin 4 is transcriptional and regulated by protein kinase C. Cell Biol. Toxicol. 16: 221–233.
Park JW, Gruys ME, McCormick K, Lee JK, Subleski J, Wigginton JM, Fenton RG, Wang JM & Wiltrout RH (2001) Primary hepatocytes from mice treated with IL-2/IL-12 produce T cell chemoattractant activity that is dependent on monokine induced by IFN-gamma (Mig) and chemokine responsive to gamma-2 (Crg-2). J. Immunol. 166: 3763–3770.
Acknowledgements
This work was supported by the BBSRC (C Willberg), the Medical Research Council (E Barnes), the Wellcome Trust and the European Union-grant code QLK2-CT-1999-00356 (P Klenerman).
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by M Piacentini
Rights and permissions
About this article
Cite this article
Willberg, C., Barnes, E. & Klenerman, P. HCV immunology–Death and the maiden T cell. Cell Death Differ 10 (Suppl 1), S39–S47 (2003). https://doi.org/10.1038/sj.cdd.4401122
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/sj.cdd.4401122
Keywords
This article is cited by
-
Mechanisms of Disease: the evolving understanding of liver allograft rejection
Nature Clinical Practice Gastroenterology & Hepatology (2008)
-
Hepatitis C: virus, host, disease
Cell Death & Differentiation (2003)