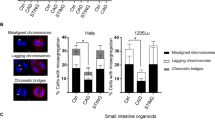
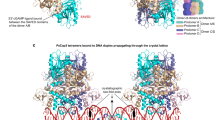
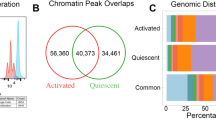
Abstract
Apoptosis is often accompanied by degradation of chromosomal DNA. CAD, caspase-activated DNase, was identified in 1998 as a DNase that is responsible for this process. In the last several years, mice deficient in the CAD system have been generated. Studies with these mice indicated that apoptotic DNA degradation occurs in two different systems. In one, the DNA fragmentation is carried out by CAD in the dying cells and in the other, by lysosomal DNase II after the dying cells are phagocytosed. Several other endonucleases have also been suggested as candidate effectors for the apoptotic degradation of chromosomal DNA. In this review, we will discuss the mechanism and role of DNA degradation during apoptosis.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- CAD:
-
caspase-activated DNase
- ICAD:
-
inhibitor of CAD
- DFF:
-
DNA fragmentation factor
References
Jacobson MD, Weil M and Raff MC (1997) Programmed cell death in animal development. Cell 88: 347–354
Evan G and Littlewood T (1998) A matter of life and cell death. Science 281: 1317–1322
Nagata S (1997) Apoptosis by death factor. Cell 88: 355–365
Ashkenazi A and Dixit VM (1998) Death receptors: signaling and modulation. Science 281: 1305–1308
Green DR and Reed JC (1998) Mitochondria and apoptosis. Science 281: 1309–1312
Kerr JF, Wyllie AH and Currie AR (1972) Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer 26: 239–257
Fadok VA, Bratton DL, Frasch SC, Warner ML and Henson PM (1998) The role of phosphatidylserine in recognition of apoptotic cells by phagocytes. Cell Death Differ. 5: 551–562
Savill J and Fadok V (2000) Corpse clearance defines the meaning of cell death. Nature 407: 784–788
Thornberry NA and Lazebnik Y (1998) Caspases: enemies within. Science 281: 1312–1316
Samali A, Zhivotovsky B, Jones D, Nagata S and Orrenius S (1999) Apoptosis: cell death defined by caspase activation. Cell Death Differ. 6: 495–496
Wyllie AH (1980) Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature 284: 555–556
Lagarkova MA, Iarovaia OV and Razin SV (1995) Large-scale fragmentation of mammalian DNA in the course of apoptosis proceeds via excision of chromosomal DNA loops and their oligomers. J. Biol. Chem. 270: 20239–20241
Oberhammer F, Wilson JW, Dive C, Morris ID, Hickman JA, Wakeling AE, Walker PR and Sikorska M (1993) Apoptotic death in epithelial cells: cleavage of DNA to 300 and/or 50 kb fragments prior to or in the absence of internucleosomal fragmentation. EMBO J. 12: 3679–3684
Khodarev NN, Bennett T, Shearing N, Sokolova I, Koudelik J, Walter S, Villalobos M and Vaughan AT (2000) LINE L1 retrotransposable element is targeted during the initial stages of apoptotic DNA fragmentation. J. Cell Biochem. 79: 486–495
Sun XM and Cohen GM (1994) Mg(2+)-dependent cleavage of DNA into kilobase pair fragments is responsible for the initial degradation of DNA in apoptosis. J. Biol. Chem. 269: 14857–14860
Cohen JJ, Duke RC, Fadok VA and Sellins KS (1992) Apoptosis and programmed cell death in immunity. Annu. Rev. Immunol. 10: 267–293
Peitsch MC, Polzar B, Stephan H, Crompton T, MacDonald HR, Mannherz HG and Tschopp J (1993) Characterization of the endogenous deoxyribonuclease involved in nuclear DNA degradation during apoptosis (programmed cell death). EMBO J. 12: 371–377
Barry MA and Eastman A (1993) Identification of deoxyribonuclease II as an endonuclease involved in apoptosis. Arch. Biochem. Biophys. 300: 440–450
Montague JW, Hughes FJ and Cidlowski JA (1997) Native recombinant cyclophilins A, B, and C degrade DNA independently of peptidylprolyl cis–trans-isomerase activity. Potential roles of cyclophilins in apoptosis. J. Biol. Chem. 272: 6677–6684
Shiokawa D, Iwamatsu A and Tanuma S (1997) Purification, characterization and amino acid sequencing of DNase γ from rat spleen. Arch. Biochem. Biophys. 346: 15–20
Napirei M, Karsunky H, Zevnik B, Stephan H, Mannherz HG and Moroy T (2000) Features of systemic lupus erythematosus in DNase1-deficient mice. Nat. Genet. 25: 177–181
Nicholson DW, Ali A, Thornberry NA, Vaillancourt JP, Ding CK, Gallant M, Gareau Y, Griffin PR, Labelle M, Lazebnik YA, Munday NA, Raju SM, Smulson ME, Yamin T-T, Yu VL and Miller DK (1995) Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature 376: 37–43
Chow SC, Weis M, Kass GEN, Holmstrom TH, Eriksson JE and Orrenius S (1995) Involvement of multiple proteases during Fas-mediated apoptosis in T lymphocytes. FEBS Lett. 364: 134–138
Dubrez L, Savoy I, Hamman A and Solary E (1996) Pivotal role of a DEVD-sensitive step in etoposide-induced and Fas-mediated apoptotic pathways. EMBO J. 15: 5504–5512
Enari M, Hug H and Nagata S (1995) Involvement of an ICE-like protease in Fas-mediated apoptosis. Nature 375: 78–81
Enari M, Hase A and Nagata S (1995) Apoptosis by a cytosolic extract from Fas-activated cells. EMBO J. 14: 5201–5208
Martin SJ, Newmeyer DD, Mathias S, Farschon DM, Wang H-G, Reed JC, Kolesnik RN and Green DR (1995) Cell-free reconstitution of Fas-, UV radiation- and ceramide-induced apoptosis. EMBO J. 14: 5191–5200
Janicke RU, Sprengart ML, Wati MR and Porter AG (1998) Caspase-3 Is required for DNA fragmentation and morphological changes associated with apoptosis. J. Biol. Chem. 273: 9357–9360
Enari M, Talanian RV, Wong WW and Nagata S (1996) Sequential activation of ICE-like and CPP32-like proteases during Fas-mediated apoptosis. Nature 380: 723–726
Liu X, Zou H, Slaughter C and Wang X (1997) DFF, a heterodimeric protein that functions downstream of caspase-3 to trigger DNA fragmentation during apoptosis. Cell 89: 175–184
Mitamura S, Ikawa H, Mizuno N, Kaziro Y and Itoh H (1998) Cytosolic nuclease activated by caspase-3 and inhibited by DFF-45. Biochem. Biophys. Res. Commun. 243: 480–484
Enari M, Sakahira H, Yokoyama H, Okawa K, Iwamatsu A and Nagata S (1998) A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature 391: 43–50
Halenbeck R, MacDonald H, Roulston A, Chen TT, Conroy L and Williams LT (1998) CPAN, a human nuclease regulated by the caspase-sensitive inhibitor DFF45. Curr. Biol. 8: 537–540
Liu X, Li P, Widlak P, Zou H, Luo X, Garrard WT and Wang X (1998) The 40-kDa subunit of DNA fragmentation factor induces DNA fragmentation and chromatin condensation during apoptosis. Proc. Natl. Acad. Sci. USA 95: 8461–8466
Mukae N, Enari M, Sakahira H, Fukuda Y, Inazawa J, Toh H and Nagata S (1998) Molecular cloning and characterization of human caspase-activated DNase. Proc. Natl. Acad. Sci. USA 95: 9123–9128
Yokoyama H, Mukae N, Sakahira H, Okawa K, Iwamatsu A and Nagata S (2000) A novel activation mechanism of caspase-activated DNase from Drosophila melanogaster. J. Biol. Chem. 275: 12978–12986
Sakahira H, Enari M and Nagata S (1999) Functional differences of two forms of the inhibitor of caspase- activated DNase, ICAD-L, and ICAD-S. J. Biol. Chem. 274: 15740–15744
Widlak P, Li P, Wang X and Garrard WT (2000) Cleavage preferences of the apoptotic endonuclease DFF40 (caspase-activated DNase or nuclease) on naked DNA and chromatin substrates. J. Biol. Chem. 275: 8226–8232
Sakahira H, Takemura Y and Nagata S (2001) Enzymatic active site of caspase-activated DNase (CAD) and its inhibition by inhibitor of CAD (ICAD). Arch. Biochem. Biophys. 388: 91–99
Liu X, Zou H, Widlak P, Garrard W and Wang X (1999) Activation of the apoptotic endonuclease DFF40 (caspase-activated DNase or nuclease). Oligomerization and direct interaction with histone H1. J. Biol. Chem. 274: 13836–13840
Meiss G, Scholz SR, Korn C, Gimadutdinow O and Pingoud A (2001) Identification of functionally relevant histidine residues in the apoptotic nuclease CAD. Nucleic Acids Res. 29: 3901–3909
Korn C, Scholz SR, Gimadutdinow O, Pingoud A and Meiss G (2002) Involvement of conserved histidine, lysine and tyrosine residues in the mechanism of DNA cleavage by the caspase-3 activated DNase CAD. Nucleic Acids Res. 30: 1325–1332
Durrieu F, Samejima K, Fortune JM, Kandels-Lewis S, Osheroff N and Earnshaw WC (2000) DNA topoisomerase IIalpha interacts with CAD nuclease and is involved in chromatin condensation during apoptotic execution. Curr. Biol. 10: 923–926
Sakahira H, Enari M and Nagata S (1998) Cleavage of CAD inhibitor in CAD activation and DNA degradation during apoptosis. Nature 391: 96–99
Gu J, Dong RP, Zhang C, McLaughlin DF, Wu MX and Schlossman SF (1999) Functional interaction of DFF35 and DFF45 with caspase-activated DNA fragmentation nuclease DFF40. J. Biol. Chem. 274: 20759–20762
Sakahira H and Nagata S (2002) Co-translational folding of caspase-activated DNase with Hsp70, Hsp40 and inhibitor of caspase-activated DNase. J. Biol. Chem. 277: 3364–3370
Zhang J, Liu X, Scherer DC, van Kaer L, Wang X and Xu M (1998) Resistance to DNA fragmentation and chromatin condensation in mice lacking the DNA fragmentation factor 45. Proc. Natl. Acad. Sci. USA 95: 12480–12485
Mukae N, Yokoyama H, Yokokura T, Sakoyama Y, Sakahira H and Nagata S (2000) Identification and developmental expression of inhibitor of caspase-activated DNase (ICAD) in Drosophila melanogaster. J. Biol. Chem. 275: 21402–21408
Chen D, Stetler RA, Cao G, Pei W, O'Horo C, Yin XM and Chen J (2000) Characterization of the rat DNA fragmentation factor 35/Inhibitor of caspase-activated DNase (Short form). The endogenous inhibitor of caspase-dependent DNA fragmentation in neuronal apoptosis. J. Biol. Chem. 275: 38508–38517
McIlroy D, Sakahira H, Talanian RV and Nagata S (1999) Involvement of caspase 3-activated DNase in internucleosomal DNA cleavage induced by diverse apoptotic stimuli. Oncogene 18: 4401–4408
Wolf BB, Schuler M, Echeverri F and Green DR (1999) Caspase-3 is the primary activator of apoptotic DNA fragmentation via DNA fragmentation factor-45/inhibitor of caspase-activated DNase inactivation. J. Biol. Chem. 274: 30651–30656
McCarty JS, Toh SY and Li P (1999) Study of DFF45 in its role of chaperone and inhibitor: two independent inhibitory domains of DFF40 nuclease activity. Biochem. Biophys. Res. Commun. 264: 176–180
McCarty JS, Toh SY and Li P (1999) Multiple domains of DFF45 bind synergistically to DFF40: roles of caspase cleavage and sequestration of activator domain of DFF40. Biochem. Biophys. Res. Commun. 264: 181–185
Woo M, Hakem R, Soengas MS, Duncan GS, Shahinian A, Kagi D, Hakem A, McCrrach M, Khoo W, Kaufman SA, Senaldi G, Howard T, Lowe SW and Mak TW (1998) Essential contribution of caspase 3/CPP32 to apoptosis and its associated nuclear changes. Genes Dev. 12: 806–819
Tang D and Kidd VJ (1998) Cleavage of DFF-45/ICAD by multiple caspases is essential for its function during apoptosis. J. Biol. Chem. 273: 28549–28552
Thomas DA, Du C, Xu M, Wang X and Ley T (2000) DFF45/ICAD can be directly processed by granzyme B during the induction of apoptosis. Immunity 12: 621–632
Sharif-Askari E, Alam A, Rheaume E, Beresford PJ, Scotto C, Sharma K, Lee D, DeWolf WE, Nuttall ME, Lieberman J and Sekaly RP (2001) Direct cleavage of the human DNA fragmentation factor-45 by granzyme B induces caspase-activated DNase release and DNA fragmentation. EMBO J. 20: 3101–3113
Inohara N, Koseki T, Chen S, Wu X and Nunez G (1998) CIDE, a novel family of cell death activators with homology to the 45 kDa subunit of the DNA fragmentation factor. EMBO J. 17: 2526–2533
Otomo T, Sakahira H, Uegaki K, Nagata S and Yamazaki T (2000) Structure of the heterodimeric complex between CAD domains of CAD and ICAD. Nat. Struct. Biol. 7: 658–662
Uegaki K, Otomo T, Sakahira H, Shimizu M, Yumoto N, Kyogoku Y, Nagata S and Yamazaki T (2000) Structure of the CAD domain of caspase-activated DNase and interaction with the CAD domain of its inhibitor. J. Mol. Biol. 297: 1121–1128
Zhou P, Lugovskoy AA, McCarty JS, Li P and Wagner G (2001) Solution structure of DFF40 and DFF45 N-terminal domain complex and mutual chaperone activity of DFF40 and DFF45. Proc. Natl. Acad. Sci. USA 98: 6051–6055
Samejima K and Earnshaw WC (2000) Differential localization of ICAD-L and ICAD-S in cells due to removal of a C-terminal NLS from ICAD-L by alternative splicing. Exp. Cell Res. 255: 314–320
Lechardeur D, Drzymala L, Sharma M, Zylka D, Kinach R, Pacia J, Hicks C, Usmani N, Rommens JM and Lukacs GL (2000) Determinants of the nuclear localization of the heterodimeric DNA fragmentation factor (ICAD/CAD). J. Cell Biol. 150: 321–334
Zhivotovsky B, Samali A, Gahm A and Orrenius S (1999) Caspases: their intracellular localization and translocation during apoptosis. Cell Death Differ. 6: 644–651
Nagata T, Kishi H, Liu QL, Matsuda T, Imanaka T, Tsukada K, Kang D and Muraguchi A (2002) The regulation of DNAse activities in subcellular compartments of activated thymocytes. Immunology 105: 399–406
Xerri L, Palmerini F, Devilard E, Defrance T, Bouabdallah R, Hassoun J and Birg F (2000) Frequent nuclear localization of ICAD and cytoplasmic co-expression of caspase-8 and caspase-3 in human lymphomas. J. Pathol. 192: 194–202
Samejima K and Earnshaw WC (1998) ICAD/DFF regulator of apoptotic nuclease is nuclear. Exp. Cell Res. 243: 453–459
Rosl F (1992) A simple and rapid method for detection of apoptosis in human cells. Nucleic Acids Res. 20: 5243
Gavrieli Y, Sherman Y and Ben-Sasson SA (1992) Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J. Cell Biol. 119: 493–501
Staley K, Blaschke A and Chun J (1997) Apoptotic DNA fragmentation is detected by a semi-quantitative ligation-mediated PCR of blunt DNA ends. Cell Death Differ. 4: 66–75
Wu YC, Stanfield GM and Horvitz HR (2000) NUC-1, a Caenorhabditis elegans DNase II homolog, functions in an intermediate step of DNA degradation during apoptosis. Genes Dev. 14: 536–548
Kawane K, Fukuyama H, Adachi M, Sakahira H, Copeland NG, Gilbert DJ, Jenkin NA and Nagata S (1999) Structure and promoter analysis of murine CAD and ICAD genes. Cell Death Differ. 6: 745–752
McIlroy D, Tanaka M, Sakahira H, Fukuyama H, Suzuki M, Yamamura K-I, Ohsawa Y and Uchiyama Y, Nagata S (2000) An auxiliary mode of apoptotic DNA fragmentation provided by phagocytes. Genes Dev. 14: 549–558
Boulares AH, Zoltoski AJ, Yakovlev A, Xu M and Smulson ME (2001) Roles of DNA fragmentation factor and poly(ADP-ribose) polymerase in an amplification phase of tumor necrosis factor-induced apoptosis. J. Biol. Chem. 276: 38185–38192
Samejima K, Tone S and Earnshaw WC (2001) CAD/DFF40 nuclease is dispensable for high molecular weight DNA cleavage and stage I chromatin condensation in apoptosis. J. Biol. Chem. 276: 45427–45432
Zhang J, Wang X, Bove K and Xu M (1999) DNA fragmentation factor 45-deficient cells are more resistant to apoptosis and exhibit different dying morphology than wild-type control cells. J. Biol. Chem. 274: 37450–37454
Patterson SD, Spahr CS, Daugas E, Susin SA, Irinopoulou T, Koehler C and Kroemer G (2000) Mass spectrometric identification of proteins released from mitochondria undergoing permeability transition. Cell Death Differ. 7: 137–144
Li LY, Luo X and Wang X (2001) Endonuclease G is an apoptotic DNase when released from mitochondria. Nature 412: 95–99
van Loo G, Schotte P, van Gurp M, Demol H, Hoorelbeke B, Gevaert K, Rodriguez I, Ruiz-Carrillo A, Vandekerckhove J, Declercq W, Beyaert R and Vandenabeele P (2001) Endonuclease G: a mitochondrial protein released in apoptosis and involved in caspase-independent DNA degradation. Cell Death Differ. 8: 1136–1142
Ruiz-Carrillo A and Renaud J (1987) Endonuclease G: a (dG)n X (dC)n-specific DNase from higher eukaryotes. EMBO J. 6: 401–407
Widlak P, Li LY, Wang X and Garrard WT (2001) Action of recombinant human apoptotic endonuclease G on naked DNA and chromatin substrates: cooperation with exonuclease and DNase I. J. Biol. Chem. 276: 48404–48409
Parrish J, Li L, Klotz K, Ledwich D, Wang X and Xue D (2001) Mitochondrial endonuclease G is important for apoptosis in C. elegans. Nature 412: 90–94
Sakahira H, Enari M, Ohsawa Y, Uchiyama Y, Nagata S (1999) Apoptotic nuclear morphological change without DNA fragmentation. Curr. Biol. 9: 543–546
Zhang J, Lee H, Lou DW, Bovin GP and Xu M (2000) Lack of obvious 50 kilobase pair DNA fragments in DNA fragmentation factor 45-deficient thymocytes upon activation of apoptosis. Biochem. Biophys. Res. Commun. 274: 225–229
Susin SA, Lorenzo HK, Zamzami N, Marzo I, Snow BE, Brothers GM, Mangion J, Jacotot E, Costantini P, Loeffler M, Larochette N, Goodlett DR, Aebersold R, Siderovski DP, Penninger JM and Kroemer G (1999) Molecular characterization of mitochondrial apoptosis-inducing factor. Nature 397: 441–446
Susin SA, Daugas E, Ravagnan L, Samejima K, Zamzami N, Loeffler M, Costantini P, Ferri KF, Irinopoulou T, Prevost MC, Brothers G, Mak TW, Penninger J, Earnshaw WC and Kroemer G (2000) Two distinct pathways leading to nuclear apoptosis. J. Exp. Med. 192: 571–580
Stroh C and Schulze-Osthoff K (1998) Death by a thousand cuts: an ever increasing list of caspase substrates. Cell Death Differ. 5: 997–1000
Zhang J, Lee H, Agarwala A, Wen Lou D and Xu M (2001) DNA fragmentation factor 45 mutant mice exhibit resistance to kainic acid-induced neuronal cell death. Biochem. Biophys. Res. Commun. 285: 1143–1149
Slane JM, Lee HS, Vorhees CV, Zhang J and Xu M (2000) DNA fragmentation factor 45 deficient mice exhibit enhanced spatial learning and memory compared to wild-type control mice. Brain Res. 867: 70–79
Yakovlev AG, Di X, Movsesyan V, Mullins PG, Wang G, Boulares H, Zhang J, Xu M and Faden AI (2001) Presence of DNA fragmentation and lack of neuroprotective effect in DFF45 knockout mice subjected to traumatic brain injury. Mol. Med. 7: 205–216
Odaka C and Mizuochi T (2002) Macrophages are involved in DNA degradation of apoptotic cells in murine thymus after administration of hydrocortisone. Cell Death Differ. 9: 104–112
Hanayama R, Tanaka M, Miwa K, Shinohara A, Iwamatsu A and Nagata S (2002) Identification of a factor that links apoptotic cells to phagocytes. Nature 417: 182–187
Bernardi G (1971) Spleen acid deoxyribonuclease. In The Enzymes Boyer PD, ed. New York and London: Academic Press, pp 271–287
Kawane K, Fukuyama H, Kondoh G, Takeda J, Ohsawa Y, Uchiyama Y and Nagata S (2001) Requirement of DNase II for definitive erythropoiesis in the mouse fetal liver. Science 292: 1546–1549
Krieser RJ, MacLea KS, Longnecker DS, Fields JL, Fiering S and Eastman A (2002) Deoxyribonuclease IIa is required during the phagocytic phase of apoptosis and its loss causes lethality. Cell Death Differ. 9: 956–962
Hedgecock EM, Sulston JE and Thomson JN (1983) Mutations affecting programmed cell deaths in the nematode Caenorhabditis elegans. Science 220: 1277–1279
Odaka C and Mizuochi T (1999) Role of macrophage lysosomal enzymes in the degradation of nucleosomes of apoptotic cells. J. Immunol. 163: 5346–5352
Leadbetter EA, Rifkin IR, Hohlbaum AM, Beaudette BC, Shlomchik MJ and Marshak-Rothstein A (2002) Chromatin–IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature 416: 603–607
Aliprantis AO, Diez-Roux G, Mulder LC, Zychlinsky A and Lang RA (1996) Do macrophages kill through apoptosis? Immunol. Today 17: 573–576
Diez-Roux G and Lang RA (1997) Macrophages induce apoptosis in normal cells in vivo. Development 124: 3633–3638
Hoeppner DJ, Hengartner MO and Schnabel R (2001) Engulfment genes cooperate with ced-3 to promote cell death in Caenorhabditis elegans. Nature 412: 202–206
Reddien PW, Cameron S and Horvitz HR (2001) Phagocytosis promotes programmed cell death in C. elegans. Nature 412: 198–202
Cao G, Pei W, Lan J, Stetler RA, Luo Y, Nagayama T, Graham SH, Yin XM, Simon RP and Chen J (2001) Caspase-activated DNase/DNA fragmentation factor 40 mediates apoptotic DNA fragmentation in transient cerebral ischemia and in neuronal cultures. J. Neurosci. 21: 4678–4690
Nagase H, Fukuyama H, Tanaka M, Kawane K and Nagata S (2002) Mutually regulated expression of caspase-activated DNase and its inhibitor for apoptotic DNA fragmentation. Cell Death Differ. (in press).
Mukae N, Yokoyama H, Yokokura T, Sakoyama Y and Nagata S (2002) Activation of the innate immunity in Drosophila by endogenous chromosomal DNA that escaped apoptotic degradation. Genes & Develop 16: 2662–2671
Acknowledgements
The work in our laboratory was supported in part by Grants-in-aid from the Ministry of Education, Science, Sports, and Culture in Japan. KK and NM were supported by a Research fellowship of the Japan Society for the Promotion of Science. We thank Drs. L Migletta and G Gray of Clarity Editing for the careful editing of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by M Hengartner
Rights and permissions
About this article
Cite this article
Nagata, S., Nagase, H., Kawane, K. et al. Degradation of chromosomal DNA during apoptosis. Cell Death Differ 10, 108–116 (2003). https://doi.org/10.1038/sj.cdd.4401161
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/sj.cdd.4401161
Keywords
This article is cited by
-
The mechanism of HMGB1 secretion and release
Experimental & Molecular Medicine (2022)
-
Potent anticancer activity of a novel iridium metallodrug via oncosis
Cellular and Molecular Life Sciences (2022)
-
Comparative analysis of the effects of opioids in angiogenesis
BMC Anesthesiology (2021)
-
Deciphering the molecular pathways of apoptosis using purified fractions from leaf extract of Basella alba through studying the regulation of apoptosis related genes
Molecular Biology Reports (2021)
-
Nitric oxide–inducing Genistein elicits apoptosis-like death via an intense SOS response in Escherichia coli
Applied Microbiology and Biotechnology (2020)