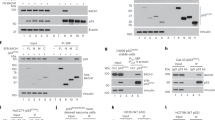
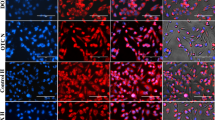
Abstract
The mechanism of p53-dependent apoptosis is still only partly defined. Using early-passage embryonic fibroblasts (MEF) from wild-type (wt), p53−/− and bax−/− mice, we observe a p53-dependent translocation of Bax to the mitochondria and a release of mitochondrial Cytochrome c during stress-induced apoptosis. These events proceed independent of zVAD-inhibitable caspase activation, are not prevented by dominant negative FADD (DN-FADD), but are negatively regulated by Mdm-2. Bcl-xL expression prevents the release of mitochondrial Cytochrome c and apoptosis, but not Bax translocation. At a single-cell level, enforced expression of p53 is sufficient to induce Bax translocation and Cytochrome c release. Real-time RT-PCR analysis reveals a significant induction of RNA expression of Noxa and Bax in p53+/+, but not in p53−/− MEF. Noxa protein expression becomes detectable prior to Bax translocation, and downregulation of endogenous Noxa by RNA interference protects wt MEF against p53-dependent apoptosis. Hence, in oncogene-expressing MEF p53 induces apoptosis by BH3 protein-dependent caspase activation.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- DMEM:
-
Dulbecco's modified essential medium
- FADD:
-
Fas-associated death domain
- MEF:
-
murine embryonic fibroblasts
- PARP:
-
poly(ADP-ribose) polymerase
- PCR:
-
polymerase chain reaction
- RNAi:
-
RNA interference
- TMRE:
-
tetramethyl-rhodamine-ethylester
- zVAD-fmk:
-
benzyloxycarbonyl-Val-Ala-Asp-fluoromethyl ketone
References
Vogelstein B, Lane D and Levine AJ (2000) Surfing the p53 network. Nature 408: 307–310
Sherr CJ and DePinho RA (2000) Cellular senescence: mitotic clock or culture shock. Cell 102: 407–410
Vousden KH (2000) p53: death star. Cell 103: 691–694
Donehower LA, Harvey M, Slagle BL, McArthur MJ, Montgomery Jr. CA, Butel JS and Bradley A (1992) Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature 356: 215–221
Lowe SW, Ruley HE, Jacks T and Housman DE (1993) p53- dependent apoptosis modulates the cytotoxicity of anticancer agents. Cell 74: 957–967
Serrano M, Lee H-W, Chin L, Cordon-Cardo C, Beach D and DePinho RA (1996) Role of the INK4a Locus in tumor suppression and cell mortality. Cell 85: 27–37
Kamijo T, Zindy F, Roussel MF, Quelle DE, Downing HR, Ashmun RA, Grosveld G and Sherr CJ (1997) Tumor suppression at the mouse INK4a locus mediated by the alternative reading frame product p19ARF. Cell 91: 649–659
Eischen CM, Weber JD, Roussel MF, Sherr CJ and Cleveland HL (1999) Disruption of the ARF–Mdm2–p53 tumor suppressor pathway in Myc-induced lymphomagenesis. Genes Dev. 13: 2658–2669
Chin L, Tam L, Pomerantz J, Wong M, Holash J, Bardeesy N, Shen Q, O'Hagan R, Pantginis J, Zhou H, Horner II JW, Cordon-Cardo C, Yancopoulos GD and DePinho RA (1999) Essential role for oncogenic Ras in tumor maintenance. Nature 400: 468–472
Schmitt CA, Fridman JS, Yang M, Lee S, Baranov E, Hoffman RM and Lowe SW (2002) A senescence program controlled by p53 and p16 INK4a contributes to the outcome of cancer therapy. Cell 109: 335–346
Hollstein M, Rice K, Greenblatt MS, Soussi T, Fuchs R, Sorlie T, Hovig E, Smith Sorensen B, Montesano R and Harris CC (1994) Database of p53 gene somatic mutations in human tumors and cell lines. Nucleic Acids Res. 22: 3551–3555
Owen-Schaub LB, Zhang W, Cusack JC, Angelo LS, Santee SM, Fujiwara T, Roth JA, Deisseroth AB, Zhang WW, Kruzel E and Radinsky R (1995) Wild-type human p53 and a temperature-sensitive mutant induce Fas/APO-1 expression. Mol. Cell. Biol. 15: 3032–3040
Müller M, Wilder S, Bannasch D, Israeli D, Lehlbach K, Li-Weber M, Friedman SL, Galle PR, Stremmel W, Oren M and Krammer PH (1998) p53 activates the CD95 (APO-1/Fas) gene in response to DNA damage by anticancer drugs. J. Exp. Med. 188: 2033–2045
Wu GS, Burns TF, McDonald III ER, Jiang W, Meng R, Krantz ID, Kao G, Gan DD, Zhou JY, Muschel R, Hamilton SR, Spinner NB, Markowitz SD, Wu G and El-Deiry WS (1997) KILLER/DR5 is a DNA damage-inducible p53-regulated death receptor gene. Nat. Genet. 17: 141–143
Miyashita T and Reed HC (1995) Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell 80: 293–299
Oda E, Ohki R, Murasawa H, Nemoto J, Shibue T, Yamashita R, Tokino T, Taniguchi T and Tanaka N (2000) Noxa, a BH3-only member of the Bcl-2 family and candidate mediator of p53-induced apoptosis. Science 288: 1053–1058
Yu J, Zhang L, Hwang PM, Kinzler KW and Vogelstein B (2001) PUMA induces the rapid apoptosis of colorectal cancer cells. Mol. Cell 7: 673–682
Nakano K and Vousden KH (2001) PUMA, a novel proapoptotic gene, is induced by p53. Mol. Cell 7: 683–694
Polyak K, Xia Y, Zweier JL, Kinzler KW and Vogelstein B (1997) A model for p53 induced apoptosis. Nature 389: 300–305
Lin Y, Ma W and Benchimol S (2000) Pidd, a new death-domain containing protein, is induced by p53 and promotes apoptosis. Nat. Genet. 26: 122–127
Buckbinder L, Talbott R, Velasco-Miguel S, Takenaka I, Faha B, Seizinger BR and Kley N (1995) Induction of the growth inhibitor IGF-binding protein 3 by p53. Nature 377: 646–649
Attardi LD, Reczek EE, Cosmas C, Demicco EG, McCurrach ME, Lowe SW and Jacks T (2000) PERP, an apoptosis-associated target of p53, is a novel member of the PMP-22/gas3 family. Genes Dev. 14: 704–718
Oda K, Arkawa H, Tanaka T, Matsuda K, Tanikawa C, Mori T, Nishimori H, Tamai K, Tokino T, Nakamura Y and Taya Y (2000) p53AIP1, a potential mediator of p53-dependent apoptosis, and its regulation by Ser-46-phosphorylated p53. Cell 102: 849–862
Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES and Wang X (1997) Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates and apoptotic protease cascade. Cell 91: 479–489
Du C, Fang M, Li Y, Li L and Wang X (2000) Smac, a mitochondrial protein that promotes Cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell 102: 33–42
Verhagen AM, Ekert PG, Pakusch M, Silke J, Connolly KM, Reid GE, Moritz RL, Simpson RJ and Vaux DL (2000) Identification of DIABLO, a mammalian protein that promotes apoptosis by binding to and antagonizing IAP proteins. Cell 102: 43–53
Suzuki Y, Imai Y, Nakayama H, Takahashi K, Takio K and Takahashi R (2001) A serine protease, HtrA2, is released from the mitochondria and interacts with XIAP, inducing cell death. Mol. Cell 8: 613–621
Hedge R, Srinivasula SM, Zhang Z, Wassell R, Mukattash R, Cilenti I, DuBois G, Lazebnik YA, Zervos AS, Fernandes-Alnemri T and Alnemri ES (2002) Identification of Omi/HtrA2 as a mitochondrial apoptotic serine protease that disrupts IAP-caspase interaction. J. Biol. Chem. 277: 432–438
Martins LM, Iaccarino I, Tenev T, Gschmeissner S, Totty NF, Lemoine NR, Savopoulos J, Gray CW, Creasy CL, Dingwall C and Downward J (2002) The serine protease Omi/HtrA2 regulates apoptosis by binding XIAP through a Reaper-like motif. J. Biol. Chem. 277: 439–444
Verhagen AM, Silke J, Ekert PG, Pakusch M, Kaufmann H, Connolly LM, Day CL, Tikoo A, Burke R, Wrobel C, Moritz RL, Simpson RJ and Vaux DL (2002) HtrA2 promotes cell death through its serine protease activity and its ability to antagonise inhibitor of apoptosis proteins. J. Biol. Chem. 277: 445–454
Green DR and Reed JC (1998) Mitochondria and apoptosis. Science 281: 1309–1312
Luo X, Budihardjo I, Zou H, Slaughter C and Wang X (1998) Bid, a Bcl-2 interacting protein, mediates Cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell 94: 481–490
Li H, Zhou H, Xu C-J and Yuan J (1988) Cleavage BID by Caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell 94: 491–501
Gross A, Yin X-M, Wang K, Wei MC, Jockel J, Milliman C, Erdjument-Bromage H, Tempst P and Korsmeyer SJ (1999) Caspase cleaved BID targets mitochondria and is required for Cytochrome c release, while BCL-XL prevents this release but not tumor necrosis factor-R1/Fas death. J. Biol. Chem. 274: 1156–1163
Yin X-M, Wang K, Gross A, Zhao Y, Zinkel S, Klocke B, Roth KA and Korsmeyer SJ (1999) Bid-deficient mice are resistant to Fas-induced hepatocellular apoptosis. Nature 400: 886–891
Hsu Y-T, Wolter KG and Youle RJ (1997) Cytosol-to-membrane redistribution of Bax and Bcl-xL during apoptosis. Proc. Natl. Acad. Sci. USA 94: 3668–3672
Deshager S, Osen-Sand A, Nichols A, Eskes R, Montessuit S, Lauper S, Maundrell K, Antonsson B and Martinou J-C (1999) Bid-induced conformational change of Bax is responsible for mitochondrial Cytochrome c release during apoptosis. J. Cell Biol. 144: 891–901
Nomura M, Shimizu S, Ito T, Narita M, Matsuda H and Tsujimoto Y (1999) Apoptotic cytosol facilitates Bax translocation to mitochondria that involves cytosolic factor regulated by Bcl-2. Cancer Res. 59: 5542–5548
Putcha GV, Deshmukh M and Johnson Jr. EM (2000) Inhibition of apoptotic signaling cascades causes loss of trophic factor dependence during neuronal maturation. J. Cell Biol. 149: 1011–1017
Perez D and White E (2000) TNF-a signals apoptosis through a bid-dependent conformational change in Bax that is inhibited by E1B 19 K. Mol. Cell 6: 53–63
O'Connor L, Strasser A, O'Reilly LA, Hausmann G, Adams JM, Cory S and Huang DCS (1998) Bim: a novel member of the Bcl-2 family that promotes apoptosis. EMBO J. 17: 384–395
Puthalakath H, Huang DCS, O'Reilly LA, King SM and Strasser A (1999) The proapoptotic activity of the Bcl-2 family member bim is regulated by interaction with the dynein motor complex. Mol. Cell 3: 287–296
Schuler M, Bossy-Wetzel E, Goldstein JC, Fitzgerald P and Green DR (2000) p53 induces apoptosis by caspase activation through mitochondrial Cytochrome c release. J. Biol. Chem. 275: 7337–7342
Li K, Li Y, Shelton JM, Richardson JA, Spencer E, Chen ZJ, Wang X and Williams RS (2000) Cytochrome c deficiency causes embryonic lethality and attenuates stress-induced apoptosis. Cell 101: 389–399
Yoshida H, Kong Y-Y, Yoshida R, Elia AJ, Hakem A, Hakem R, Penninger JM and Mak TW (1998) Apaf-1 is required for mitochondrial pathways of apoptosis and brain development. Cell 94: 739–750
Cecconi F, Alvarez-Bolado G, Meyer BI, Roth KA and Gruss P (1988) Apaf-1 (CED-4 Homolog) regulates programmed cell death in mammalian development. Cell 94: 727–737
Soengas MS, Alarcón RM, Yoshida H, Giaccia AJ, Hakem R, Mak TW and Lowe SW (1999) Apaf-1 and Caspase-9 in p53-dependent apoptosis and tumor inhibition. Science 284: 156–159
Hakem R, Hakem A, Duncan GS, Henderson JT, Woo M, Soengas MS, Elia A, de al Pompa JL, Kagi D, Khoo W, Potter J, Yoshoda R, Kaufman SA, Lowe SW and Mak TW (1998) Differential requirement for Caspase 9 in apoptotic pathways in vivo. Cell 94: 339–352
Kuida K, Haydar TF, Kuan C-Y, Gu Y, Taya C, Karasuyama H, Su MSS, Rakic P and Flavell RA (1998) Reduced apoptosis and Cytochrome c-mediated caspase activation in mice lacking Caspase 9. Cell 94: 325–337
Knudson CM, Tung KSK, Tourtellotte WG, Brown GAJ and Korsmeyer SJ (1995) Bax-deficient mice with lymphoid hyperplasia and male germ cell death. Science 270: 96–99
McCurrach ME, Connor TMF, Knudson CM, Korsmeyer SJ and Lowe SW (1997) Bax-deficiency promotes drug resistance and oncogenic transformation by attenuating p53-dependent apoptosis. Proc. Natl. Acad. Sci. USA 94: 2345–2349
Sabbatini P, Han J, Chiou SK, Nicholson DW and White E (1997) Interleukin 1b converting enzyme-like proteases are essential for p53-mediated transcriptionally dependent apoptosis. Cell Growth Differ. 8: 643–653
Zhang L, Yu J, Park BH, Kinzler KW and Vogelstein B (2000) Role of BAX in the apoptotic response to anticancer agents. Science 290: 989–992
Juin P, Hueber A-O, Littlewood T and Evan G (1999) c-Myc-induced sensitization to apoptosis is mediated through cytochrome c release. Genes Dev. 13: 1367–1381
Jürgensmeier JM, Xie A, Deveraux Q, Ellerby L, Bredesen D and Reed JC (1998) Bax directly induces release of cytochrome c from isolated mitochondria. Proc. Natl. Acad. Sci. USA 95: 4997–5002
Finucane DM, Bossy-Wetzel E, Waterhouse NJ, Cotter TG and Green DR (1999) Bax-induced caspase activation and apoptosis via Cytochrome c release from mitochondria is inhibitable by Bcl-xL. J. Biol. Chem. 274: 2225–2233
Green DR (1998) Apoptotic pathways: roads to ruin. Cell 94: 695–698
Nechushtan A, Smith CL, Hsu Y-T and Youle RJ (1999) Conformation of the Bax C-terminus regulates subcellular location and cell death. EMBO J. 18: 2330–2341
Eskes R, Desagher S, Antonsson B and Martinou J-C (2000) Bid induces the oligomerization and insertion of Bax into the outer mitochondrial membrane. Mol. Cell. Biol. 20: 929–935
Suzuki M, Youle RJ and Tjandra N (2000) Structure of Bax: coregulation of dimer formation and intracellular localization. Cell 103: 645–654
Ding H-F, Lin Y-L, McGill G, Juo P, Zhu H, Blenis J, Yuan J and Fisher DE (2000) Essential role for Caspase-8 in transcription-independent apoptosis triggered by p53. J. Biol. Chem. 275: 38905–38911
Kovar H, Jug G, Printz D, Bartl S, Schmid G and Werierska-Gadek J (2000) Characterization of distinct consecutive phases in non-genotoxic p53-induced apoptosis of Ewing tumor cells and the rate-limiting role of caspase 8. Oncogene 19: 4096–4107
Feng Gao C, Ren S, Zhang L, Nakajima T, Ichinose S, Hara T, Koike K and Tsuchida N (2001) Caspase-dependent cytosolic release of Cytochrome c and membrane translocation of Bax in p53-induced apoptosis. Exp. Cell Res. 265: 145–151
Oren M (1999) Regulation of the p53 tumor suppressor protein. J. Biol. Chem. 274: 36031–36034
Duelli DM and Lazebnik YA (2000) Primary cells suppress oncogene-dependent apoptosis. Nat. Cell Biol. 2: 859–862
Li P-F, Dietz R and von Harsdorf R (1999) p53 regulates mitochondrial membrane potential through reactive oxygen species and induces cytochrome c-independent apoptosis blocked by Bcl-2. EMBO J. 18: 6027–6036
Goldstein JC, Waterhouse NJ, Juin P, Evan GI and Green DR (2000) The coordinate release of cytochrome c during apoptosis is rapid, complete and kinetically invariant. Nat. Cell Biol. 2: 156–162
Wolter KG, Hsu Y-T, Smith CL, Nechushtan A, Xi XG and Youle RJ (1997) Movement of Bax from the cytosol to mitochondria during apoptosis. J. Cell Biol. 139: 1281–1292
Relaix F, Wei X, Li W, Pan J, Lin Y, Bowtell DD, Sassoon DA and Wu X (2000) Pw1/Peg3 is a potential cell death mediator and cooperates with Siah 1a in p53-mediated apoptosis. Proc. Natl. Acad. Sci. USA 97: 2105–2110
El-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, Lin D, Mercer WE, Kinzler K and Vogelstein B (1993) WAF1, a potential mediator of p53 tumor suppression. Cell 75: 817–825
Lindsten T, Ross AJ, King A, Zong W-X, Rathmell JC, Shiels HA, Ulrich E, Waymire KG, Mahar P, Frauwirth K, Chen Y, Wei M, Eng VM, Adelman DM, Simon MC, Ma A, Golden JA, Evan G, Korsmeyer SJ, MacGregor GR and Thompson CB (2000) The combined functions of proapoptotic Bcl-2 family members Bak and Bax are essential for normal development of multiple tissues. Mol. Cell 6: 1389–1399
Wei MC, Zong W-X, Cheng EHY, Lindsten T, Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB and Korsmeyer SJ (2001) Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science 292: 727–730
Paddison PJ and Hannon GJ (2002) RNA interference: the new somatic cell genetics. Cancer Cell 2: 17–23
Brummelkamp TR, Bernards R and Agami R (2002) A system for stable expression of short interfering RNAs in mammalian cells. Science 296: 550–553
Brummelkamp TR, Bernards R and Agami R (2002) Stable suppression of tumorigenicity by virus-mediated RNA interference. Cancer Cell 2: 243–247
Cheng EHYA, Wei MC, Weiler S, Flavell RA, Mak TW, Lindsten T and Korsmeyer SJ (2001) BCL-2, BCL-XL sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Mol. Cell 8: 705–711
Deng Y and Wu X (2000) Peg3/Pw1 promotes p53-mediated apoptosis by inducing Bax translocation from cytosol to mitochondria. Proc. Natl. Acad. Sci. USA 97: 12050–12055
Huang DCS and Strasser A (2001) BH3-only proteins – essential initiators of apoptotic cell death. Cell 103: 839–842
Zong W-X, Lindsten T, Ross AJ, MacGregor GR and Thompson CB (2001) BH3-only proteins that bind pro-survival Bcl-2 family members fail to induce apoptosis in the absence of Bax and Bak. Genes Dev. 15: 1481–1486
Caelles C, Heimberg A and Karin M (1994) p53-dependent apoptosis in the absence of transcriptional activation of p53-target genes. Nature 370: 220–223
Haupt Y, Rowan S, Shaulian E, Vousden KH and Oren M (1995) Induction of apoptosis in Hela cells by transactivation-deficient p53. Genes Dev. 9: 2170–2183
Chen X, Ko LJ, Jayaraman L and Prives C (1996) p53 levels, functional domains, and DNA damage determine the extent of the apoptotic response of tumor cells. Genes Dev. 10: 2438–2451
Serrano M, Lin AW, McCurrach ME, Beach D and Lowe SW (1997) Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 88: 593–602
Acknowledgements
We are indebted to Dr. SJ Korsmeyer for providing cells from Bax-deficient mice, to Dr. T Lin for animal care and to P Fitzgerald for help with cloning. Drs. SW Lowe, GJ Hannon, T Kitamura, M Oren, S Hedrick, DC Maneval, RJ Youle and R Agami are thanked for providing expression vectors.
This work was supported by National Institutes of Health grants GM52735, CA69381, and AI40646 awarded to DRG, and by the Max-Eder-Programm of the Deutsche Krebshilfe to MS (70-2952).
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by G Melino
Rights and permissions
About this article
Cite this article
Schuler, M., Maurer, U., Goldstein, J. et al. p53 triggers apoptosis in oncogene-expressing fibroblasts by the induction of Noxa and mitochondrial Bax translocation. Cell Death Differ 10, 451–460 (2003). https://doi.org/10.1038/sj.cdd.4401180
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/sj.cdd.4401180
Keywords
This article is cited by
-
Deletion of the BH3-only protein Noxa alters electrographic seizures but does not protect against hippocampal damage after status epilepticus in mice
Cell Death & Disease (2017)
-
Deregulated BCL-2 family proteins impact on repair of DNA double-strand breaks and are targets to overcome radioresistance in lung cancer
Journal of Cancer Research and Clinical Oncology (2017)
-
Anti-tumor effect of β-glucan from Lentinus edodes and the underlying mechanism
Scientific Reports (2016)
-
Myeloid leukemia factor 1 interfered with Bcl-XL to promote apoptosis and its function was regulated by 14-3-3
Journal of Physiology and Biochemistry (2015)
-
Impact of human papilloma virus infection on the response of head and neck cancers to anti-epidermal growth factor receptor antibody therapy
Cell Death & Disease (2014)