Abstract
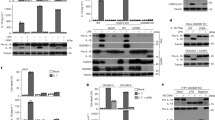
The potassium ionophore nigericin induces cell death and promotes the maturation and release of IL-1β in lipopolysaccharide (LPS)-primed monocytes and macrophages, the latter depending on caspase-1 activation by an unknown mechanism. Here, we investigate the pathway that triggers cell death and activates caspase-1. We show that without LPS priming, nigericin alone triggered caspase-1 activation and IL-18 generation in THP-1 monocytic cells. Simultaneously, nigericin induced caspase-1-independent necrotic cell death, which was blocked by the cathepsin B inhibitor CA-074-Me and other cathepsin inhibitors. Cathepsin B activation after nigericin treatment was determined biochemically and corroborated by rapid lysosomal leakage and translocation of cathepsin B to the cytoplasm. IL-18 maturation was prevented by both caspase-1 and cathepsin B inhibitors in THP-1 cells, primary mouse macrophages and human blood monocytes. Moreover, IL-18 generation was reduced in THP-1 cells stably transformed either with cystatin A (an endogenous cathepsin inhibitor) or antisense cathepsin B cDNA. Collectively, our study establishes a critical role for cathepsin B in nigericin-induced caspase-1-dependent IL-18 maturation and caspase-1-independent necrosis.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- pro-IL-1β:
-
prointerleukin-1β
- pro-IL-18:
-
prointerleukin-18
- LPS:
-
lipopolysaccharide
- ICE:
-
IL-1β-converting enzyme
- TLR:
-
Toll-like receptor
- ALLN:
-
Ac-Leu-Leu-Asn aldehyde
- zYVAD-cmk:
-
z-Tyr-Val-Ala-Asp-chloromethylketone
- zDEVD-fmk:
-
z-Asp(OMe)-Glu(OMe)-Val-DL-Asp(OMe) fluoromethylketone
- zVAD-fmk:
-
z-Val-Ala-DL-Asp-fluoromethylketone
- CA-074:
-
L-trans-epoxysuccinyl-Ile-Pro-OH propylamide
- zVF:
-
z-Val-Phe-aldehyde
- zLLL:
-
z-Leu-Leu-Leu-aldehyde
- CA-074-Me:
-
L-trans-epoxysuccinyl-Ile-Pro-OMe propylamide
- zDEVD-afc:
-
z-Asp-Glu-Val-DL-Asp-7-amino- 4-trifluoromethylcoumarin
- zYVAD-afc:
-
N-acetyl-Tyr-Val-Ala-Asp-7-amino -4-trifluoromethylcoumarin
- zRR-amc:
-
z-Arg-Arg-7-amino-4-methylcoumarin
- zFR-amc:
-
z-Phe-Arg-7-amino-4-methylcoumarin
- CHAPS:
-
3-([-3-cholamidopropyl) dimethylammonio]-1-propanesulfonic acid
- PMA:
-
phorbol-myristate acetate; MNC, mononuclear cells
References
Dinarello CA (1997) Interleukin-1. Cytokine Growth Factor Rev. 8: 253
Dinarello CA (1999) Interleukin-18. Methods 19: 121–132
Nakanishi K, Yoshimoto T, Tsutsui H and Okamura H (2001) Interleukin-18 regulates both Th1 and Th2 responses. Annu. Rev. Immunol. 19: 423–474
Puren AJ, Fantuzzi G and Dinarello CA (1999) Gene expression, synthesis, and secretion of interleukin 18 and interleukin 1β are differentially regulated in human blood mononuclear cells and mouse spleen cells. Proc. Natl. Acad. Sci. USA 96: 2256–2261
Lin XY, Choi MS and Porter AG (2000) Expression analysis of the human caspase-1 sub-family reveals specific regulation of the casp-5 gene by lipopolysaccharide and interferon-γ. J. Biol. Chem. 275: 39920–39926
Zheng TS, Hunot S, Kuida K and Flavell RA (1999) Caspase knockouts: matters of life and death. Cell Death Differ. 6: 1043–1053
Wang J and Lenardo MJ (2000) Roles of caspases in apoptosis, development, and cytokine maturation revealed by homozygous gene deficiencies. J. Cell Sci. 113: 753–757
Stevenson FT, Torrano F, Locksley RM and Lovett DH (1992) Interleukin 1: the patterns of translation and intracellular distribution support alternative secretory mechanisms. J. Cell Physiol. 152: 223–231
Wewers MD, Dare HA, Winnard AV, Parker JM and Miller DK (1997) IL-1β-converting enzyme (ICE) is present and functional in human alveolar macrophages: macrophage IL-1β release limitation is ICE independent. J. Immunol. 159: 5964–5972
Hogquist KA, Nett MA, Unanue ER and Chaplin DD (1991) Interleukin 1 is processed and released during apoptosis. Proc. Natl. Acad. Sci. USA 88: 8485–8489
Perregaux D, Barberia J, Lanzetti AJ, Geoghegan KF, Carty TJ and Gabel CA (1992) IL-1β maturation: evidence that mature cytokine formation can be induced specifically by nigericin. J. Immunol. 149: 1294–1303
Perregaux D and Gabel CA (1994) Interleukin-1β maturation and release in response to ATP and nigericin. Evidence that potassium depletion mediated by these agents is a necessary and common feature of their activity. J. Biol. Chem. 269: 15195–15203
Walev I, Reske K, Palmer M, Valeva A and Bhakdi S (1995) Potassium-inhibited processing of IL-1β in human monocytes. EMBO J. 14: 1607–1614
Cheneval D, Ramage P, Kastelic T, Szelestenyi T, Niggli H, Hemmig R, Bachmann M and MacKenzie A (1998) Increased mature interleukin-1β (IL-1β) secretion from THP-1 cells induced by nigericin is a result of activation of p45 IL-1β-converting enzyme processing. J. Biol. Chem. 273: 17846–17851
Laliberte RE, Eggler J and Gabel CA (1999) ATP treatment of human monocytes promotes caspase-1 maturation and externalization. J. Biol. Chem. 274: 36944–36951
Jonas D, Walev I, Berger T, Liebetrau M, Palmer M and Bhakdi S (1994) Novel path to apoptosis: small transmembrane pores created by staphylococcal alpha-toxin in T lymphocytes evoke internucleosomal DNA degradation. Infect. Immun. 62: 1304–1312
Warny M and Kelly CP (1999) Monocytic cell necrosis is mediated by potassium depletion and caspase-like proteases. Am. J. Physiol. 276: C717–C724
Bantel H, Sinha B, Domschke W, Peters G, Schulze-Osthoff K and Janicke RU (2001) alpha-Toxin is a mediator of Staphylococcus aureus-induced cell death and activates caspases via the intrinsic death pathway independently of death receptor signaling. J. Cell Biol. 155: 637–648
Wang S, Miura M, Jung YK, Zhu H, Li E and Yuan J (1998) Murine caspase-11, an ICE-interacting protease, is essential for the activation of ICE. Cell 92: 501–509
Martinon F, Burns K and Tschopp J (2002) The inflammasome. A molecular platform triggering activation of inflammatory caspases and processing of proIL-1β. Mol. Cell 10: 417–426
Vancompernolle K, Van Herreweghe F, Pynaert G, Van de Craen M, De Vos K, Totty N, Sterling A, Fiers W, Vandenabeele P and Grooten J (1998) Atractyloside-induced release of cathepsin B, a protease with caspase-processing activity. FEBS Lett. 438: 150–158
Schotte P, Van Criekinge W, Van de Craen M, Van Loo G, Desmedt M, Grooten J, Cornelissen M, De Ridder L, Vandekerckhove J, Fiers W, Vandenabeele P and Beyaert R (1998) Cathepsin B-mediated activation of the proinflammatory caspase-11. Biochem. Biophys. Res. Commun. 251: 379–387
Roberts LR, Adjei PN and Gores GJ (1999) Cathepsins as effector proteases in hepatocyte apoptosis. Cell. Biochem. Biophys. 30: 71–88
Foghsgaard L, Wissing D, Mauch D, Lademann U, Bastholm L, Boes M, Elling F, Leist M and Jaattela M (2001) Cathepsin B acts as a dominant execution protease in tumor cell apoptosis induced by tumor necrosis factor. J. Cell Biol. 153: 999–1010
Guicciardi ME, Miyoshi H, Bronk SF and Gores GJ (2001) Cathepsin B knockout mice are resistant to tumor necrosis factor-α-mediated hepatocyte apoptosis and liver injury: implications for therapeutic applications. Am. J. Pathol. 159: 2045–2054
Nakayama M, Ishidoh K, Kayagaki N, Kojima Y, Yamaguchi N, Nakano H, Kominami E, Okumura K and Yagita H (2002) Multiple pathways of TWEAK-induced cell death. J. Immunol. 168: 734–743
Taniguchi M, Nagaoka K, Kunikata T, Kayano T, Yamauchi H, Nakamura S, Ikeda M, Orita K and Kurimoto M (1997) Characterization of anti-human interleukin-18 (IL-18)/interferon-γ-inducing factor (IGIF) monoclonal antibodies and their application in the measurement of human IL-18 by ELISA. J. Immunol. Methods. 206: 107–113
Akita K, Ohtsuki T, Nukada Y, Tanimoto T, Namba M, Okura T, Takakura-Yamamoto R, Torigoe K, Gu Y, Su MSS, Fujii M, Satoh-Itoh M, Yamamoto K, Kohno K, Ikeda M and Kurimoto M (1997) Involvement of caspase-1 and caspase-3 in the production and processing of mature human interleukin 18 in monocytic THP-1 cells. J. Biol. Chem. 272: 26595–26603
Yamin TT, Ayala JM and Miller DK (1996) Activation of the native 45-kDa precursor form of interleukin-1-converting enzyme. J. Biol. Chem. 271: 13273–13282
Poltorak A, He X, Smirnova I, Liu MY, Huffel CV, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B and Beutler B (1998) Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282: 2085–2088
Watanabe N, Kawaguchi M and Kobayashi Y (1998) Activation of interleukin-1β-converting enzyme by nigericin is independent of apoptosis. Cytokine 10: 645–653
Zhuang J, Dinsdale D and Cohen GM (1998) Apoptosis, in human monocytic THP1 cells, results in the release of cytochrome c from mitochondria prior to their ultracondensation, formation of outer membrane discontinuities and reduction in inner membrane potential. Cell Death Differ. 5: 953–962
Livingston DJ (1997) In vitro and in vivo studies of ICE inhibitors. J. Cell Biochem. 64: 19–26
Rabuffetti M, Sciorati C, Tarozzo G, Clementi E, Manfredi AA and Beltramo M (2000) Inhibition of caspase-1-like activity by Ac-Tyr-Val-Ala-Asp-chloromethyl ketone induces long-lasting neuroprotection in cerebral ischemia through apoptosis reduction and decrease of proinflammatory cytokines. J. Neurosci. 20: 4398–4404
Schotte P, Declercq W, Van Huffel S, Vandenabeele P and Beyaert R (1999) Non-specific effects of methyl ketone peptide inhibitors of caspases. FEBS Lett. 442: 117–121
Buttle DJ, Murata M, Knight CG and Barrett AJ (1992) CA074 methyl ester: a proinhibitor for intracellular cathepsin B. Arch. Biochem. Biophys. 299: 377–380
Zhu DM and Uckun FM (2000) Cathepsin inhibition induces apoptotic death in human leukemia and lymphoma cells. Leuk. Lymphoma. 39: 343–354
Hentze H, Schwoebel F, Lund S, Kehl M, Ertel W, Wendel A, Jaattela M and Leist M (2001) In vivo and in vitro evidence for extracellular caspase activity released from apoptotic cells. Biochem. Biophys. Res. Commun. 283: 1111–1117
Wang KK (2000) Calpain and caspase: can you tell the difference? Trends Neurosci. 23: 20–26
Coolican SA, Haiech J and Hathaway DR (1986) The role of subunit autolysis in activation of smooth muscle Ca2+-dependent proteases. J. Biol. Chem. 261: 4170–4176
McCelland P, Lash JA and Hathaway DR (1989) Identification of major autolytic cleavage sites in the regulatory subunit of vascular calpain II. A comparison of partial amino-terminal sequences to deduced sequence from complementary DNA. J. Biol. Chem. 264: 17428–17431
Wang KK, Nath R, Posner A, Raser KJ, Buroker-Kilgore M, Hajimohammadreza I, Probert Jr. AW, Marcoux FW, Ye Q, Takano E, Hatanaka M, Maki M, Caner H, Collins JL, Fergus A, Lee KS, Lunney EA, Hays SJ and Yuen P (1996) An alpha-mercaptoacrylic acid derivative is a selective nonpeptide cell-permeable calpain inhibitor and is neuroprotective. Proc. Natl. Acad. Sci. USA 93: 6687–6692
Stoka V, Turk B, Schendel SL, Kim TH, Cirman T, Snipas SJ, Ellerby LM, Bredesen D, Freeze H, Abrahamson M, Bromme D, Krajewski S, Reed JC, Yin XM, Turk V and Salvesen GS (2001) Lysosomal protease pathways to apoptosis: cleavage of Bid, not pro-caspases, is the most likely route. J. Biol. Chem. 276: 3149–3157
Mehta VB, Hart J and Wewers MD (2000) ATP-stimulated release of IL-1β and IL-18 requires priming by LPS and is independent of caspase-1 cleavage. J. Biol. Chem. 276: 3820–3826
Le Feuvre RA, Brough D, Iwakura Y, Takeda K and Rothwell NJ (2002) Priming of macrophages with lipopolysaccharide potentiates P2X7-mediated cell death via a caspase-1-dependent mechanism, independently of cytokine production. J. Biol. Chem. 277: 3210–3218
Furlong IJ, Ascaso R, Lopez Rivas A and Collins MK (1997) Intracellular acidification induces apoptosis by stimulating ICE-like protease activity. J. Cell. Sci. 110: 653–661
Li P, Allen H, Banerjee S, Franklin S, Herzog L, Johnston C, McDowell J, Paskind M, Rodman L, Salfeld J, Towne E, Tracey D, Wardwell S, Wei F-Y, Wong W, Kamen R and Seshadri T (1995) Mice deficent in IL-1β-converting enzyme are defective in production of mature IL-1β and resistant to endotoxic shock. Cell 80: 401–411
Nett-Fiordalisi M, Tomaselli K, Russell JH and Chaplin DD (1995) Macrophage apoptosis in the absence of active interleukin-1β-converting enzyme. J. Leukoc. Biol. 58: 717–724
McGrath ME (1999) The lysosomal cysteine proteases. Annu. Rev. Biophys. Biomol. Struct. 28: 181–204
Turk V, Turk B and Turk D (2001) Lysosomal cysteine proteases: facts and opportunities. EMBO J. 20: 4629–4633
Reinheckel T, Deussing J, Roth W and Peters C (2001) Towards specific functions of lysosomal cysteine peptidases: phenotypes of mice deficient for cathepsin B or cathepsin L. Biol. Chem. 382: 735–741
Leist M and Jaattela M (2001) Triggering apoptosis by cathepsins. Cell Death Differ. 8: 324–326
Johnson DE (2000) Noncaspase proteases in apoptosis. Leukemia 14: 1695–1703
Foghsgaard L, Lademann U, Wissing D, Poulsen B and Jaattela M (2002) Cathepsin B mediates tumor necrosis factor-induced arachidonic acid release in tumor cells. J. Biol. Chem. 277: 39499–39506
Morland B and Pedersen A (1979) Cathepsin B activity in stimulated mouse peritoneal macrophages. Lab. Invest. 41: 379–384
Li Q, Falkler WA and Bever CT (1997) Endotoxin induces increased intracellular cathepsin B activity in THP-1 cells. Immunopharmacol. Immunotoxicol. 19: 215–237
Beutler B (2000) Tlr4: central component of the sole mammalian LPS sensor. Curr. Opin. Immunol. 12: 20–26
Leist M, Single B, Castoldi AF, Kuhnle S and Nicotera P (1997) Intracellular adenosine triphosphate (ATP) concentration: a switch in the decision between apoptosis and necrosis. J. Exp. Med. 185: 1481–1486
Bergmeyer HU (1983) Methods of Enzymatic Analysis, Vol 3. Weinheim: Verlag Chemie. 605pp.
Thornberry NA (1994) Interleukin-1β converting enzyme. Meth. Enzymol. 244: 615–631
Acknowledgements
This work was supported by the Institute of Molecular and Cell Biology, Singapore and A*STAR. AGP is an adjunct staff member of the Department of Surgery, National University of Singapore. We are grateful to Dr. Junying Yuan (Harvard Medical School) for caspase-1 antibody, Dr. Jean-Paul Klein (Institut National de la Santé et de la Recherche Médicale U932, France) for THP-1 cells, Dr. Marja Jäättelä for the cathepsin B antisense plasmid, and to Dr. C Volbracht for reviewing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by S. Nagata
Rights and permissions
About this article
Cite this article
Hentze, H., Lin, X., Choi, M. et al. Critical role for cathepsin B in mediating caspase-1-dependent interleukin-18 maturation and caspase-1-independent necrosis triggered by the microbial toxin nigericin. Cell Death Differ 10, 956–968 (2003). https://doi.org/10.1038/sj.cdd.4401264
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/sj.cdd.4401264
Keywords
This article is cited by
-
Spatiotemporal proteomics uncovers cathepsin-dependent macrophage cell death during Salmonella infection
Nature Microbiology (2020)
-
Lactobacillus-derived extracellular vesicles enhance host immune responses against vancomycin-resistant enterococci
BMC Microbiology (2017)
-
Imipramine blocks acute silicosis in a mouse model
Particle and Fibre Toxicology (2017)
-
Intranasal Delivery of a Caspase-1 Inhibitor in the Treatment of Global Cerebral Ischemia
Molecular Neurobiology (2017)
-
NLRP3 inflammasome inhibition is disrupted in a group of auto-inflammatory disease CAPS mutations
Nature Immunology (2016)