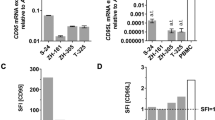
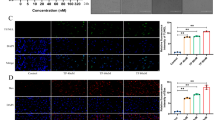
Abstract
Death receptor-mediated apoptosis of human malignant glioma cells triggered by CD95 ligand (CD95L) or Apo2 ligand/tumor necrosis factor-related apoptosis-inducing ligand (Apo2L/TRAIL) share several features, including processing of multiple caspases and mitochondrial cytochrome c release. We here report that CD95L-induced cell death is inhibited by sulfasalazine (SS) in all of four human glioma cell lines, both in the absence and presence of cycloheximide (CHX). Coexposure to CD95L and SS prevents the CD95L-evoked processing of caspases 2, 3, 8 and 9, the release of cytochrome c from mitochondria, and the loss of BCL-xL protein. This places the protective effect of SS proximal to most known events triggered by the CD95-dependent signaling pathway in glioma cells. CD95L promotes the accumulation of nuclear factor kappa B (NF-κB) in the nucleus and induces the DNA-binding activity of NF-κB assessed by electrophoretic mobility shift assay. The total levels of p50, p65 and IκBα remain unchanged, but the levels of phosphorylated IκBα and of nuclear p65 increase, in response to CD95L. IκBα phosphorylation as well as nuclear NF-κB translocation and DNA binding are blocked by SS. However, unlike SS, dominant-negative IκBα (IκBdn) does not block apoptosis, suggesting that SS inhibits CD95L-mediated apoptosis in an NF-κB-independent manner. In contrast to CD95L, the cytotoxic effects of Apo2L/TRAIL are enhanced by SS, and SS facilitates Apo2L/TRAIL-evoked caspase processing, cytochrome c release, and nuclear translocation of p65. These effects of SS are nullified in the presence of CHX, suggesting that the effects of SS and CHX are redundant or that enhanced apoptosis mediated by SS requires protein synthesis. IκBdn fails to modulate Apo2L/TRAIL-induced apoptosis. Similar effects of SS on CD95L- and Apo2L/TRAIL-induced apoptosis are observed in MCF-7 breast and HCT116 colon carcinoma cells. Interestingly, HCT cells lacking p21 (80S14p21−/−) are only slightly protected by SS from CD95L-induced apoptosis, but sensitized to Apo2L/TRAIL-induced apoptosis, indicating a link between the actions of SS and p21. Thus, SS modulates the death cascades triggered by CD95L and Apo2L/TRAIL in opposite directions in an NF-κB-independent manner, and SS may be a promising agent for the augmentation of Apo2L/TRAIL-based cancer therapies.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- Apo2L/TRAIL:
-
Apo2 ligand/tumor necrosis factor-related apoptosis-inducing ligand
- ATP:
-
adenosinetriphosphate
- CD95L:
-
CD95 ligand
- CHX:
-
cycloheximide
- DISC:
-
death-inducing signaling complex
- DR:
-
death receptor
- DTT:
-
dithiothreitol
- EMSA:
-
electrophoretic mobility shift assay
- FADD:
-
Fas-associated death domain
- FLIP:
-
FLICE-inhibitory proteins
- IκB:
-
inhibitory kappa B
- IκBdn:
-
dominant negative IκB
- IL:
-
interleukin
- NF-κB:
-
nuclear factor kappa B
- P-IκB:
-
phosphorylated IκB
- ROI:
-
reactive oxygen intermediates
- SS:
-
sulfasalazine
- TNF:
-
tumor necrosis factor
- TRAIL-R1–4:
-
TRAIL receptor 1–4
References
Ashkenazi M, Yamada T, Ichijo H, Delia D, Miyazono K, Fukumoro K and Mizutani S (1999) Death receptors: signaling and modulation. EMBO J. 18: 1223–1234
Krammer P (1999) CD95 (APO-1/Fas)-mediated apoptosis: live and let die. Adv. Immunol. 71: 163–210
Schulze-Osthoff K, Ferrari D, Los M, Wesselborg S and Peter M (1998) Apoptosis signaling by death receptors. Eur. J. Biochem. 254: 439–459
Svartz N (1941) Ett nytt sulfonamidpreparat. Forelopande meddelande. Nord. Med. 9: 544
Hoult JRS (1986) Pharmacological and biochemical actions of sulphasalazine. Drugs 32 (Suppl. 1): 18–26
Rhodes JM, Bartholomew TC and Jewell DP (1981) Inhibition of leukocyte motility by drugs used in ulcerative colitis. Gut 22: 642–647
Rubinstein A, Das KM, Melamed J and Murphy RA (1978) Comparative analysis of systemic immunological parameters in ulcerative colitis and idiopathic proctitis: effects of sulfasalazine in vivo and in vitro. Clin. Exp. Immunol. 33: 217–224
Fujiwara M, Mitsui K and Yamamoto I (1990) Inhibition of proliferative responses and interleukin 2 production by salazosulfapyridine and its metabolites. Jpn. J. Pharmacol. 54: 121–132
Gaginella TS and Walsh RE (1992) Sulfasalazine: multiplicity of action. Dig. Dis. Sci. 37: 801–812
Aruom OI, Wasil M, Halliwell B, Hoey BM and Butler J (1987) The scavenging of oxidants by sulphasalazine and its metabolites. A possible contribution to their anti-inflammatory effects? Biochem. Pharmacol. 36: 3739–3742
Gionchetti P, Guarnieri C, Campieri M, Bellusgi A, Brignola C, Tannone P, Miglioli M and Barbara C (1991) Scavenger effect of sulfasalazine, 5-aminosalicylic acid, and osalazine on superoxide radical generation. Dig. Dis. Sci. 36: 174–178
Allgayer H and Stenson WF (1988) A comparison of effects of sulfasalazine and its metabolites on the metabolism of endogenous vs exogenous arachidonic acid. Immunopharmacology 15: 39–46
Tornhamre S, Edenius C, Smedegard G, Sjoquist B and Lindgren JA (1989) Effects of sulfasalazine and a sulfasalazine analogue on the formation of lipoxygenase and cyclooxygenase products. Eur. J. Pharmacol. 169: 225–234
Horn H, Preclik G, Stange EF and Ditschuneit H (1991) Modulation of arachidonic acid metabolism by osalazine and other aminosalicylates in leukocytes. Scand. J. Gastroenterol. 26: 867–879
Wahl C, Liptay S, Adler G and Schmid RM (1997) Sulfasalazine: a potent and specific inhibitor of nuclear factor kappa B. J. Clin. Invest. 101: 1163–1174
Siebenlist U, Franzoso G and Brown K (1994) Structure, regulation and function of NF-kB. Ann. Rev. Cell Biol. 10: 405–455
Baeuerle PA and Baltimore D (1996) NF-kappaB: ten years after. Cell 87: 13–20
Beg AA, Finco TS, Nantermet PV and Baldwin AS (1993) Tumor necrosis factor and interleukin-1 lead to phosphorylation and loss of IkBa: a mechanism for NF-κB activation. Mol. Cell. Biol. 13: 3301–3310
Palombella V, Rando O, Goldberg A and Maniatis T (1994) The ubiquitin–proteasome pathway is required for processing the NF-kB1 precursor protein and the activation of NF-kB. Cell 78: 773–785
Heyninck K and Beyaert R (2001) Crosstalk between NF-kappaB-activating and apoptosis-inducing proteins of the TNF-receptor complex. Mol. Cell. Biol. Res. Commun. 4: 259–265
Dudley E, Hornung F, Zheng L, Scherer D, Ballard D and Lenardo M (1999) NF-kappaB regulates Fas/APO-1/CD95- and TCR-mediated apoptosis of T lymphocytes. Eur. J. Immunol. 29: 878–886
Micheau O, Lens S, Gaide O, Alevizopoulos K and Tschopp J (2001) NF-kappaB signals induce the expression of c-FLIP. Mol. Cell. Biol. 21: 5299–5305
Goke R, Goke A, Goke B and Chen Y (2000) Regulation of TRAIL-induced apoptosis by transcription factors. Cell. Immunol. 201: 77–82
Roth W, Fontana A, Trepel M, Reed J, Dichgans J and Weller M (1997) Immunochemotherapy of malignant glioma: synergistic activity of CD95 ligand and chemotherapeutics. Cancer Immunol. Immunother. 44: 55–63
Roehn TA, Wagenknecht B, Roth W, Naumann U, Gulbins E, Krammer PH, Walczak H and Weller M (2001) CCNU-dependent potentiation of TRAIL/Apo2L-induced apoptosis in human glioma cells is p53-independent but may involve enhanced cytochrome c release. Oncogene 20: 4128–4137
Wagenknecht B, Schulz JB, Gulbins E and Weller M (1998) Crm-A, bcl-2 and NDGA inhibit CD95L-induced apoptosis of malignant glioma cells at the level of caspase 8 processing. Cell Death Differ. 5: 894–900
Weller M, Frei K, Groscurth P, Krammer PH, Yonekawa Y and Fontana A (1994) Anti-Fas/APO-1 antibody-mediated apoptosis of cultured human glioma cells. Induction and modulation of sensitivity by cytokines. J. Clin. Invest. 94: 954–964
Hermisson M, Wagenknecht B, Wolburg H, Glaser T, Dichgans J and Weller M (2000) Sensitization to CD95 ligand-induced apoptosis in human glioma cells by hyperthermia involves enhanced cytochrome c release. Oncogene 19: 2338–2345
Glaser T, Wagenknecht B and Weller M (2001) Identification of p21 as a target of cycloheximide-mediated facilitation of CD95-mediated apoptosis in human malignant glioma cells. Oncogene 20: 4757–4767
Wagenknecht B, Glaser T, Naumann U, Kügler S, Isenmann S, Bähr M, Korneluk R, Liston P and Weller M (1999) Expression and biological activity of X-linked inhibitor of apoptosis (XIAP) in human malignant glioma. Cell Death Differ. 6: 370–376
Krueger A, Schmitz I, Baumann S, Krammer PH and Kirchhoff S (2001) Cellular FLICE-inhibitory protein splice variants inhibit different steps of caspase-8 activation at the CD95 death-inducing signaling complex. J. Biol. Chem. 276: 20633–20640
Weller M, Malipiero U, Rensing-Ehl A, Barr P and Fontana A (1995) Fas/APO-1 gene transfer for human malignant glioma. Cancer Res. 55: 2936–2944
Roth W, Isenmann S, Naumann U, Kugler S, Baehr M, Dichgans J, Ashkenazi A and Weller M (1999) Locoregional Apo2L/TRAIL eradicates intracranial human malignant glioma xenografts in athymic mice in the absence of neurotoxicity. Biochem. Biophys. Res. Commun. 265: 479–483
Rieger J, Naumann U, Glaser T, Ashkenazi A and Weller M (1998) APO2 ligand: a novel lethal weapon against malignant glioma? FEBS Lett. 427: 124–128
Ouaaz F, Li M and Beg AA (1999) A critical role for the RelA subunit of nuclear factor kappaB in regulation of multiple immune-response genes and in Fas-induced cell death. J. Exp. Med. 189: 999–1004
Kühnel F, Zender L, Paul Y, Tietze MK, Trautwein C, Manns M and Kubicka S (2000) NfkappaB mediates apoptosis through transcriptional activation of Fas (CD95) in adenoviral hepatitis. J. Biol. Chem. 275: 6421–6427
Hsu SC, Gavrilin MA, Lee HH, Wu CC, Han SH and Lai MZ (1999) NF-kappaB-dependent Fas ligand expression. Eur. J. Immunol. 29: 2948–2956
Kasibhatla S, Genestier L and Green DR (1999) Regulation of fas-ligand expression during activation-induced cell death in T lymphocytes via nuclear factor kappaB. J. Biol. Chem. 274: 987–992
Qin JZ, Bacon P, Chaturvedi V and Nickoloff BJ (2001) Role of NF-kappaB activity in apoptotic response of keratinocytes mediated by interferon-gamma, tumor necrosis factor-alpha, and tumor-necrosis-factor-related apoptosis-inducing ligand. J. Invest. Dermatol. 117: 898–907
Ravi R, Bedi A and Fuchs EJ (1998) CD95 (Fas)-induced caspase-mediated proteolysis of NF-kappaB. Cancer Res. 58: 882–886
Franco AV, Zhang XD, van Berkel E, Sanders JE, Zhang XY, Thomas WD, Nguyen T and Hersey P (2001) The role of NF-kappaB in TNF-related apoptosis-inducing ligand (TRAIL)-induced apoptosis of melanoma cells. J. Immunol. 166: 5337–5345
Ravi R, Bedi GC, Engstrom LW, Zeng Q, Mookerjee B, Gelinas C, Fuchs EJ and Bedi A (2001) Regulation of death receptor expression and TRAIL/Apo2L-induced apoptosis by NF-kappaB. Nat. Cell Biol. 3: 409–416
Ravi R and Bedi A (2002) Requirement of Bax for TRAIL/Apo2L- induced apoptosis of colorectal cancers: synergism with sulindac-mediated inhibition of Bcl-xL . Cancer Res. 62: 1583–1587
Harper N, Farrow SN, Kaptein A, Cohen GM and MacFarlane M (2001) Modulation of tumor necrosis factor apoptosis-inducing ligand-induced NF-kappaB activation by inhibition of apical caspases. J. Biol. Chem. 276: 34743–34752
Choi C, Kutsch O, Park J, Zhou T, Seol DW and Benveniste EN (2002) Tumor necrosis factor-related apoptosis-inducing ligand induces caspase-dependent interleukin-8 expression and apoptosis in human astroglioma cells. Mol. Cell. Biol. 22: 724–736
Schaub FJ, Han DK, Liles WC, Adams LD, Coats SA, Ramachandra RK, Seifert RA, Schwartz SM and Bowen-Pope DF (2000) Fas/FADD-mediated activation of a specific program of inflammatory gene expression in vascular smooth muscle cells. Nat. Med. 6: 790–796
Choi C, Xu X, Oh JW, Lee SJ, Gillespie GY, Park H, Jo H and Benveniste EN (2001) Fas-induced expression of chemokines in human glioma cells: involvement of extracellular signal-regulated kinase 1/2 and p38 mitogen-activated protein kinase. Cancer Res. 61: 3084–3091
Kataoka T, Budd RC, Holler N, Thome M, Martinon F, Irmler M, Burns K, Hahne M, Kennedy N, Kovacsovics M and Tschopp J (2000) The caspase-8 inhibitor FLIP promotes activation of NF-kappaB and Erk signaling pathways. Curr. Biol. 10: 640–648
Miyachi Y, Yoshioka A, Imamura S and Niwa Y (1994) Effect of sulphasalazine and its metabolites on the generation of reactive oxygen species. Gut 28: 190–195
Travis SP and Jewell DP (1994) Salicylates for ulcerative colitis—their mode of action. Pharmacol. Ther. 63: 135–161
Wagenknecht B, Gulbins E, Lang F, Dichgans J and Weller M (1997) Lipoxygenase inhibitors block CD95 ligand-mediated apoptosis of human malignant glioma cells. FEBS Lett. 409: 17–23
Streffer JR, Bitzer M, Schabet M, Dichgans J and Weller M (2001) Response of radiochemotherapy-associated cerebral edema to a phytotherapeutic agent H15. Neurology 56: 1219–1221
Glaser T, Winter S, Groscurth P, Safayhi H, Sailer ER, Ammon HP, Schabet M and Weller M (1999) Boswellic acids and malignant glioma: induction of apoptosis but no modulation of drug sensitivity. Br. J. Cancer 80: 756–765
Mundt B, Kuhnel F, Zender L, Paul Y, Tillmann H, Trautwein C, Manns MP and Kubicka S (2003) Involvement of TRAIL and its receptors in viral hepatitis. FASEB J. 17: 94–96
Ashkenazi A, Pai RC, Fong S, Leung S, Lawrence DA, Marsters SA, Blackie C, Chang L, McMurtrey AE, Hebert A, DeForge L, Koumenis IL, Lewis D, Harris L, Bussiere J, Koeppen H, Shahrokh Z and Schwall RH (1999) Safety and anti-tumor activity of recombinant soluble Apo2 ligand. J. Clin. Invest. 104: 155–162
Fulda S, Wick W, Weller M and Debatin KM (2002) Smac agonists sensitize for TRAIL/Apo2L- or anticancer drug-induced apoptosis and induce regression of human glioma xenografts. Nat. Med. 8: 808–815
Weller M, Marini AM and Paul SM (1992) Niacinamide blocks 3-acetylpyridine toxicity of cerebellar granule cells in vitro. Brain Res. 594: 160–164
Acknowledgements
This work was supported by Deutsche Forschungsgemeinschaft (We 1502/10-1) and the Fortüne program of the University of Tübingen (MH 831-0-0).
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by RA Knight
Rights and permissions
About this article
Cite this article
Hermisson, M., Weller, M. NF-κB-independent actions of sulfasalazine dissociate the CD95L- and Apo2L/TRAIL-dependent death signaling pathways in human malignant glioma cells. Cell Death Differ 10, 1078–1089 (2003). https://doi.org/10.1038/sj.cdd.4401269
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/sj.cdd.4401269
Keywords
This article is cited by
-
Sulfasalazine induces apoptosis of HBx-expressing cells in an NF-κB-independent manner
Virus Genes (2010)
-
NF-κB-independent sensitization of glioblastoma cells for TRAIL-induced apoptosis by proteasome inhibition
Oncogene (2007)
-
Novel sulfasalazine analogues with enhanced NF-kB inhibitory and apoptosis promoting activity
Apoptosis (2005)
-
Apoptosis in Gliomas: Molecular Mechanisms and Therapeutic Implications
Journal of Neuro-Oncology (2004)