Abstract
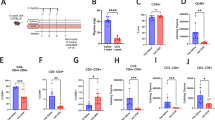
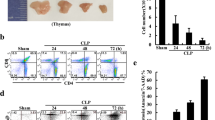
Herein, we provide the first evidence on the capsaicin (CPS) receptor vanilloid receptor type-1 (VR1) by rat thymocytes, and its involvement in CPS-induced apoptosis. VR1 mRNA was identified by quantitative RT-PCR in CD5+ thymocytes. By immunofluorescence and flow cytometry, we found that a substantial portion of CD5+ thymocytes, namely CD4+ and double negative (DN) cell subsets, express VR1 that was present on plasma membrane on discrete spots. By Western blot, VR1 protein was identified as a single band of 95 kDa. We also described that CPS could trigger two distinct pathways of thymocyte death, namely apoptosis and necrosis depending on the dose of CPS exposure. CPS-induced apoptosis involved intracellular free calcium (Ca2+) influx, phosphatidylserine exposure, mitochondrial permeability transmembrane pore (PTP) opening and mitochondrial transmembrane potential (ΔΨm) dissipation leading to cytochrome c release, activation of caspase-9 and -3 and oligonucleosomal DNA fragmentation. VR1 was functionally implicated in these events as they were completely abrogated by the VR1 antagonist, capsazepine (CPZ). Finally, we demonstrated that VR1 expression on distinct thymocytes was associated with the selective ability of CPS to trigger DNA fragmentation in VR1+ CD4+ and DN thymocytes. Overall, our results suggest that the expression of VR1 on thymocytes may function as a sensor of harmful stimuli present in the thymic environment.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- AEA:
-
anandamide
- AIF:
-
apoptose-inducing factor
- [Ca2+]:
-
intracellular free calcium
- CPS:
-
capsaicin
- CPZ:
-
capsazepine
- CSA:
-
cyclosporin A
- DEPC:
-
diethyl pyrocarbonate
- DMSO:
-
dimethylsulfoxide
- DN:
-
double negative
- DP:
-
double positive
- EDTA:
-
ethilenediaminetetra-acetic acid
- FACS:
-
fluorescent-activated cell sorter
- FCS:
-
fetal calf serum
- FDA:
-
fluorescent diacetate
- FITC:
-
fluorescein isothiocyanate
- GAPDH:
-
glyceraldehyde-3-phosphate dehydrogenase
- HRP:
-
horseradish peroxidase
- JC-1:
-
5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanineiodide
- mAb:
-
monoclonal antibody
- MFI:
-
mean fluorescence intensity
- PBMC:
-
peripheral blood mononuclear cells
- PBS:
-
phosphate-buffered saline
- PE:
-
phicoerythrin
- PI:
-
propidium iodide
- PMSF:
-
phenylmethylsulfonylfluoride
- PS:
-
phosphatidylserine
- PTP:
-
permeability transmembrane pore
- RAG:
-
rabbit anti-goat
- VR1:
-
vanilloid receptor type-1
- ΔΨm:
-
mitochondrial transmembrane potential
References
Holzer P (1991) Capsaicin: cellular targets, mechanism of action, and selectivity for thin sensory neurones. Pharmacol. Rev. 43: 143–201
Santoni G, Perfumi M, Birarelli P, Procaccini S and Piccoli M (1995) In vivo capsaicin treatment inhibits rat NK cell cytotoxic functions. Immunopharmacol. Immunotoxicol. 17: 511–528
Santoni G, Perfumi M, Bressan AM and Piccoli M (1996) Capsaicin-induced inhibition of mitogen and interleukin-2-stimulated T cell proliferation: its reversal by in vivo substance P administration. J. Neuroimmunol. 68: 131–138
Santoni G, Perfumi MC, Pompei P, Spreghini E, Lucciarini R, Martarelli D, Staffolani M and Piccoli M (2000) Impairment of rat thymocyte differentiation and functions by neonatal capsaicin treatment is associated with induction of apoptosis. J. Neuroimmunol. 104: 37–46
Santoni G, Perfumi MC, Spreghini E, Romagnoli S and Piccoli M (1999) Neurokinin type-1 receptor antagonist inhibits enhancement of T cell functions by substance P in normal and neuromanipulated capsaicin-treated rats. J. Neuroimmunol. 93: 15–25
Santoni G, Amantini C, Lucciarini R, Perfumi M, Pompei P and Piccoli M (2004) Neonatal capsaicin treatment affects rat thymocyte proliferation and cell death by modulating substance P and neurokinin-1 receptor expression. Neuroimmunomodulation 11: 160–172
Szallasi A and Blumberg PM (1999) Vanilloid (capsaicin) receptors and mechanisms. Pharmacol. Rev. 51: 159–211
Caterina MJ and Julius D (2001) The vanilloid receptor: a molecular gateway to the pain pathway. Annu. Rev. Neurosci. 24: 487–517
Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD and Julius D (1997) The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389: 816–824
Gunthorpe MJ, Benham CD, Randall A and Davis JB (2002) The diversity in the vanilloid (TRPV) receptor family of ion channels. Trends Pharmacol. Sci. 23: 183–191
Birder LA, Kanai AJ, de Groat WC, Kiss S, Nealen ML, Burke NE, Dineley KE, Watkins S, Reynolds IJ and Caterina MJ (2001) Vanilloid receptor expression suggests a sensory role for urinary bladder epithelial cells. Proc. Natl. Acad. Sci. USA 98: 13396–13401
Dvorakova M and Kummer W (2001) Transient expression of vanilloid receptor subtype 1 in rat cardiomyocytes during development. Histochem. Cell Biol. 116: 223–225
Kato S, Aihara E, Nakamura A, Xin H, Matsui H, Kohama K and Takeuchi K (2003) Expression of vanilloid receptors in rat gastric epithelial cells: role in cellular protection. Biochem. Pharmacol. 66: 1115–1121
Southall MD, Li T, Gharibova LS, Pei Y, Nicol GD and Travers JB (2003) Activation of epidermal vanilloid receptor-1 induces release of proinflammatory mediators in human keratinocytes. J. Pharmacol. Exp. Ther. 304: 217–222
Biro T, Maurer M, Modarres S, Lewin NE, Brodie C, Acs G, Acs P, Paus R and Blumberg PM (1998) Characterization of functional vanilloid receptors expressed by mast cells. Blood 91: 1332–1340
Schumacher MA, Moff I, Sudanagunta SP and Levine JD (2000) Molecular cloning of an N-terminal splice variant of the capsaicin receptor. Loss of N-terminal domain suggests functional divergence among capsaicin receptor subtypes. J. Biol. Chem. 275: 2756–2762
Clapham DE (1997) Some like it hot: spicing up ion channels. Nature 389: 783–784
Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzemburg M, Basbaum AI and Julius D (2000) Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 288: 306–313
Hail Jr N (2003) Mechanisms of vanilloid-induced apoptosis. Apoptosis 8: 251–262
Morre DJ, Chueh PJ and Morrè DM (1995) Capsaicin inhibits preferentially the NADH oxidase and growth of transformed cells in culture. Proc. Natl. Acad. Sci. USA 92: 1831–1835
Wolvetang EJ, Larm JA, Moutsoulas P and Lawen A (1996) Apoptosis induced by inhibitors of the plasma membrane NADH-oxidase involves Bcl-2 and calcineurin. Cell Growth Differ. 7: 1315–1325
Biro T, Brodie C, Modarres S, Lewin NE, Acs P and Blumberg PM (1998) Specific vanilloid responses in C6 rat glioma cells. Brain Res. Mol. 56: 89–98
Macho A, Calzado MA, Munoz-Blanco J, Gomez-Diaz C, Gajate C, Mollinedo F, Navas P and Munoz E (1999) Selective induction of apoptosis by capsaicin in transformed cells: the role of reactive oxygen species and calcium. Cell Death Differ. 6: 155–165
Jambrina E, Alonso R, Alcalde M, del Carmen Rodriguez M, Serrano A, Martinez-A C, Garcia-Sancho J and Izquierdo M (2003) Calcium influx through receptor-operated channel induces mitochondria-triggered paraptotic cell death. J. Biol. Chem. 278: 14134–14145
Zhang J, Nagasaki M, Tanaka Y and Morikawa S (2003) Capsaicin inhibits growth of adult T-cell leukemia cells. Leuk. Res. 27: 275–283
Sugimoto T, Xiao C and Ichikawa H (1998) Neonatal primary neuronal death induced by capsaicin and axotomy involves an apoptotic mechanism. Brain Res. 807: 147–154
Dedov VN, Mandadi S, Armati PJ and Verkhratsky A (2001) Capsaicin-induced depolarisation of mitochondria in dorsal root ganglion neurons is enhanced by vanilloid receptors. Neuroscience 103: 219–226
Lee YS, Nam DH and Kim JA (2000) Induction of apoptosis by capsaicin in A172 human glioblastoma cells. Cancer Lett. 161: 121–130
Macho A, Blazquez MV, Navas P and Munoz E (1998) Induction of apoptosis by vanilloid compounds does not require de novo gene transcription and activator protein 1 activity. Cell Growth Differ. 9: 277–286
Hiura A, Nakae Y and Nakagawa H (2002) Cell death of primary afferent nerve cells in neonatal mice treated with capsaicin. Anat. Sci. Int. 77: 47–50
Olah Z, Karai L and Iadarola MJ (2001) Anandamide activates vanilloid receptor 1 (VR1) at acidic pH in dorsal root ganglia neurons and cells ectopically expressing VR1. J. Biol. Chem. 276: 31163–31170
Grant ER, Dubin AE, Zhang SP, Zivin RA and Zhong Z (2002) Simultaneous intracellular calcium and sodium flux imaging in human vanilloid receptor 1 (VR1)-transfected human embryonic kidney cells: a method to resolve ionic dependence of VR1-mediated cell death. J. Pharmacol. Exp. Ther. 300: 9–17
Meddings JB, Hogaboam CM, Tran K, Reynolds JD and Wallace JL (1991) Capsaicin effects on non-neuronal plasma membranes. Biochem. Biophys. Acta 1070: 43–50
Jung MY, Kang HJ and Moon A (2001) Capsaicin-induced apoptosis in SK-Hep-1 hepatocarcinoma cells involves Bcl-2 downregulation and caspase-3 activation. Cancer Lett. 165: 139–145
Bevan S, Hothi S, Hughes G, James IF, Rang HP, Shah K, Walpole CS and Yeats JC (1992) Capsazepine: a competitive antagonist of the sensory neurone excitant capsaicin. Br. J. Pharmacol. 107: 544–552
Wood JN, Winter J, James IF, Rang HP, Yeats J and Bevan S (1988) Capsaicin-induced ion fluxes in dorsal root ganglion cells in culture. J. Neurosci. 8: 3208–3220
Inoue K, Koizumi S, Fuziwara S, Denda S, Inoue K and Denda M (2002) Functional vanilloid receptors in cultured normal human epidermal keratinocytes. Biochem. Biophys. Res. Commun. 291: 124–129
McConkey DJ and Orrenius S (1997) The role of calcium in the regulation of apoptosis. Biochem. Biophys. Res. Commun. 239: 357–366
McConkey DJ, Hartzell P, Jondal M and Orrenius S (1989) Inhibition of DNA fragmentation in thymocytes and isolated thymocyte nuclei by agents that stimulate protein kinase C. J. Biol. Chem. 264: 13399–13402
McConkey DJ, Hartzell P, Amador-Perez JF, Orrenius S and Jondal M (1989) Calcium-dependent killing of immature thymocytes by stimulation via the CD3/T cell receptor-complex. J. Immunol. 143: 1801–1806
Kroemer G and Reed JC (2000) Mitochondrial control of cell death. Nat. Med. 6: 513–519
Nicoletti I, Migliorati G, Pagliacci MC, Grignani F and Riccardi C (1991) A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J. Immunol. Methods 139: 271–279
Cohen GM (1997) Caspases: the executioners of apoptosis. Biochem. J. 326: 1–15
Scollay R and Shortman K (1983) Thymocyte subpopulations: an experimental review including flow cytometric cross-correlation between the major murine thymocyte markers. Thymus 5: 245–249
Hunig T, Torres-Nagel N, Mehling B, Park HJ and Herrmann T (2001) Thymic development and repertoire selection, the rat perspective. Immunol. Rev. 184: 7–19
Kedei N, Szabo T, Lile JD, Treanor JJ, Olah Z, Iadarola MJ and Blumberg PM (2001) Analysis of the native quaternary structure of vanilloid receptor 1. J. Biol. Chem. 276: 28613–28619
Osborne BA (1996) Apoptosis and maintenance of homeostasis in the immune system. Curr. Opin. Immunol. 8: 245–254
Quaglino D and Ronchetti IP (2001) Cell death in the rat thymus: a minireview. Apoptosis 6: 389–401
Mignotte B and Vayssiere JL (1998) Mitochondria and apoptosis. Eur. J. Biochem. 252: 1–15
Zamzami N, Marchetti P, Castedo M, Zanin C, Vayssiere JL, Petit PX and Kroemer G (1995) Reduction in mitochondrial potential constitutes an early irreversible step of programmed lymphocyte death in vivo. J. Exp. Med. 181: 1661–1672
Kroemer G, Zamzami N and Susin SA (1997) Mitochondrial control of apoptosis. Immunol. Today 18: 44–51
Andreyev A and Fiskum G (1999) Calcium induced release of mitochondrial cytochrome c by different mechanisms selective for brain versus liver. Cell Death Differ. 6: 825–832
Waring P and Beaver J (1996) Cyclosporin A rescues thymocytes from apoptosis induced by very low concentrations of thapsigargin: effects on mitochondrial function. Exp. Cell Res. 227: 264–276
Micheau O, Hammann A, Solary E and Dimanche-Boitrel MT (1999) STAT-1-Indipendent upregulation of FADD and procaspase-3 and -8 in cancer cells treated with cytotoxic drugs. Biochem. Biophys. Res. Commun. 256: 603–607
Kang HJ, Soh Y, Kim MS, Lee EJ, Surh YJ, Kim HR, Kim SH and Moon A (2003) Roles of JNK-1 and p38 in selective induction of apoptosis by capsaicin in ras-transformed human breast epithelial cells. Int. J. Cancer. 103: 475–482
Macho A, Lucena C, Calzado MA, Blanco M, Donnay I, Appendino G and Munoz E (2000) Phorboid-20-homovanillates induces apoptosis through a VR1-independent mechanism. Chem. Biol. 7: 277–286
De Petrocellis L, Bisogno T, Ligresti A, Bifulco M, Melck D and Di Marzo V (2002) Effect on cancer cell proliferation of palmitoylethanolamide, a fatty acid amide interacting with both the cannabinoid and vanilloid signalling systems. Fundam. Clin. Pharmacol. 16: 297–302
Ross RA (2003) Anandamide and vanilloid TRVP1 receptors. Br. J. Pharmacol. 140: 790–801
Hwang SW, Cho H, Kwak J, Lee SY, Kang CJ, Jung J, Cho S, Min KH, Suh YG, Kim D and Oh U (2000) Direct activation of capsaicin receptors by products of lipoxygenases: endogenous capsaicin-like substances. Proc. Natl. Acad. Sci. USA 97: 6155–6160
Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sorgard M, Di Marzo V, Julius D and Hogestatt ED (1999) Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature 400: 452–457
Schwarz H, Blanco FJ and Lotz M (1994) Anandamide, an endocannabinoid receptor agonist inhibits lymphocyte proliferation and induces apoptosis. J. Neuroimmunol. 55: 107–115
Valk P, Verbakel S, Vankan Y, Hol S, Mancham S, Ploemacher R, Mayen A, Lowenberg B and Delwel R (1997) Anandamide, a natural ligand for the peripheral cannabinoid receptor is a novel synergistic growth factor for hematopoietic cells. Blood 90: 1448–1457
Maccarrone M, Lorenzon T, Bari M, Melino G and Finazzi-Agro A (2000) Anandamide induces apoptosis in human cells via vanilloid receptors. Evidence for a protective role of cannabinoid receptors. J. Biol. Chem. 275: 31938–31945
Sellins KS and Cohen JJ (1991) Hyperthermia induces apoptosis in thymocytes. Radiat. Res. 126: 88–95
Maccarone M, Melino G and Finazzi-Agro A (2001) Lipoxygenases and their involvement in programmed cell death. Cell Death Differ. 8: 776–784
Frey T (1997) Correlated flow cytometric analysis of terminal events in apoptosis reveals the absence of some changes in some model systems. Cytometry 28: 253–263
Sandstrom K, Hakansson L, Lukinius A and Venge P (2000) A method to study apoptosis in eosinophils by flow cytometry. J. Immunol. Methods 240: 55–68
Kao JPY, Harootunian AT and Tsein RY (1989) Photochemically generated cytosolic calcium pulses and their detection by Fluo 3. J. Biol. Chem. 264: 8179–8184
Acknowledgements
This study was partially supported by Ministero dell'Università e della Ricerca Scientifica e Tecnologica (MIUR), University of Camerino.
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by B Osborne
Rights and permissions
About this article
Cite this article
Amantini, C., Mosca, M., Lucciarini, R. et al. Distinct thymocyte subsets express the vanilloid receptor VR1 that mediates capsaicin-induced apoptotic cell death. Cell Death Differ 11, 1342–1356 (2004). https://doi.org/10.1038/sj.cdd.4401506
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/sj.cdd.4401506
Keywords
This article is cited by
-
Optimized flow cytometric detection of transient receptor potential vanilloid-1 (TRPV1) in human hematological malignancies
Medical Oncology (2022)
-
New Insights on the Role of TRP Channels in Calcium Signalling and Immunomodulation: Review of Pathways and Implications for Clinical Practice
Clinical Reviews in Allergy & Immunology (2021)
-
TRPing on the pore phenomenon: what do we know about transient receptor potential ion channel-related pore dilation up to now?
Journal of Bioenergetics and Biomembranes (2016)
-
Transient Receptor Potential (TRP) channels in T cells
Seminars in Immunopathology (2016)
-
Spermatogonial stem cell sensitivity to capsaicin: An in vitro study
Reproductive Biology and Endocrinology (2008)