Abstract
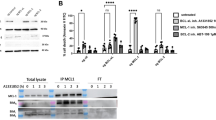
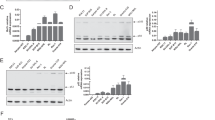
Bfl-1/A1 is generally recognized as a Bcl-2-related inhibitor of apoptosis. We show that Bfl-1 undergoes constitutive ubiquitin/proteasome-mediated turnover. Moreover, while Bfl-1 suppresses apoptosis induced by staurosporine or cytokine withdrawal, it is proapoptotic in response to tumor necrosis factor (TNF) receptor activation in FL5.12 pro-B cells. Its anti- versus proapoptotic effect is regulated by two proteolytic events: (1) its constitutive proteasome-mediated turnover and (2) its TNF/cycloheximide (CHX)-induced cleavage by μ-calpain, or a calpain-like activity, coincident with acquisition of a proapoptotic phenotype. In vitro studies suggest that calpain-mediated cleavage of Bfl-1 occurs between its Bcl-2 homology (BH)4 and BH3 domains. This would be consistent with the generation of a proapoptotic Bax-like BH1–3 molecule. Overall, our studies uncovered two new regulatory mechanisms that play a decisive role in determining Bfl-1's prosurvival versus prodeath activities. These findings might provide important clues to counteract chemoresistance in tumor cells that highly express Bfl-1.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- STS:
-
staurosporine
- TNF:
-
tumor necrosis factor
- BH:
-
Bcl-2 homology
- TM:
-
transmembrane
- CHX:
-
cycloheximide
- zVAD-fmk:
-
carbobenzoxy-Val-Ala-Asp-fluoromethyl ketone
- GFP:
-
green fluorescent protein
- ALLN:
-
N-acetyl-L-leucyl-L-leucyl-norleucinal
- ALLM:
-
N-acetyl-L-leucyl-L-leucyl-methioninal
- IL-3:
-
interleukin-3
References
Adams JM and Cory S (1998) The Bcl-2 protein family: arbiters of cell survival. Science 281: 1322–1326
Cory S and Adams JM (2002) The Bcl2 family: regulators of the cellular life-or-death switch. Nat. Rev. Cancer 2: 647–656
Petros AM, Olejniczak ET and Fesik SW (2004) Structural biology of the Bcl-2 family of proteins. Biochim. Biophys. Acta 1644: 83–94
Cheng EH, Wei M, Weiler S, Flavell RA, Mak TW, Lindsten T and Korsmeyer SJ (2001) BCL-2, BCL-XL sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Mol. Cell 8: 705–711
Letai A, Bassik MC, Walensky LD, Sorcinelli MD, Weiler S and Korsmeyer SJ (2002) Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell 2: 183–192
Chen L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds MG, Colman PM, Day CL, Adams JM and Huang DCS (2005) Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol. Cell 17: 393–403
Chittenden T (2002) BH3 domains: intracellular death-ligands critical for initiating apoptosis. Cancer Cell 2: 165–166
Walensky LD, Kung AL, Escher I, Malia TJ, Barbuto S, Wright RD, Wagner G, Verdine GL and Korsmeyer SJ (2004) Activation of apoptosis in vivo by a hydrocarbon-stapled BH3 helix. Science 305: 1466–1470
Clem RJ, Cheng EH, Karp CL, Kirsch DG, Ueno K, Takahashi A, Kastan MB, Griffin DE, Earnshaw WC, Veliuona MA and Hardwick JM (1998) Modulation of cell death by Bcl-xL through caspase interaction. Proc. Natl. Acad. Sci. USA 95: 554–559
Cheng EH, Kirsch DG, Clem RJ, Ravi R, Kastan MB, Bedi A, Ueno K and Hardwick JM (1997) Conversion of Bcl-2 to a Bax-like death effector by caspases. Science 278: 1966–1968
Nakagawa T and Yuan J (2000) Cross-talk between two cysteine protease families. Activation of caspase-12 by calpain in apoptosis. J. Cell Biol. 150: 887–894
Wang K (2000) Calpain or caspase: can you tell the difference? Trends Neurosci. 23: 20–26
Chan SL and Mattson MP (1999) Caspase and calpain substrates: roles in synaptic plasticity and cell death. J. Neurosci. Res. 58: 167–190
Choi SS, Park I-C, Yun JW, Sung YC, Hong S-I and Shin H-S (1995) A novel Bcl-2 related gene, Bfl-1, is overexpressed in stomach cancer and preferentially expressed in bone marrow. Oncogene 11: 1693–1698
Lin EY, Orlofsky A, Berger MS and Prystowsky MB (1993) Characterization of A1, a novel hemopoietic-specific early-response gene with sequence similarity to bcl-2. J. Immunol. 151: 1979–1988
Hirotani M, Zhang Y, Fujita N, Naito M and Tsuruo T (1999) NH2-terminal BH4 domain of Bcl-2 is functional for heterodimerization with Bax and inhibition of apoptosis. J. Biol. Chem. 274: 20415–20420
Zhang H, Cowan-Jacob SW, Simonen M, Greenhalf W, Heim J and Meyhack B (2000) Structural basis of Bfl-1 for its interaction with Bax and anti-apoptotic action in mammalian and yeast cells. J. Biol. Chem. 275: 11092–11099 (a) Sedlak TW, Oltvai ZN, Yang E, Wang K, Boise LH, Thompson CB and Korsmeyer SJ (1995) Multiple Bcl-2 family members demonstrate selective dimerizations with Bax. Proc. Natl. Acad. Sci. USA 92: 7834–7838
D’Sa-Eipper C and Chinnadurai G (1998) Functional dissection of Bfl-1, a Bcl-2 homolog: anti-apoptosis, oncogene-cooperation and cell proliferation activities. Oncogene 16: 3105–3114
Hatakeyama S, Hamasaki A, Negishi I, Loh DY, Sendo F and Nakayama K (1998) Multiple gene duplication and expression of mouse bcl-2-related genes, A1. Int. Immunol. 10: 631–637
Ko JK, Lee MJ, Cho SH, Cho JA, Lee BY, Koh JS, Lee SS, Shim YH and Kim CW (2003) Bfl-1S, a novel alternative splice variant of Bfl-1, localizes in the nucleus via its C-terminus and prevents cell death. Oncogene 22: 2457–2465
Kenny JJ, Knobloch TJ, Augustus M, Carter KC, Rosen CA and Lang JC (1997) GRS, a novel member of the Bcl-2 gene family, is highly expressed in multiple cancer cell lines and in normal leukocytes. Oncogene 14: 997–1001
Zong WX, Edelstein LC, Chen C, Bash J and Gélinas C (1999) The prosurvival Bcl-2 homolog Bfl-1/A1 is a direct transcriptional target of NF-κB that blocks TNFα-induced apoptosis. Genes Dev. 13: 382–387
Hu X, Yee E, Harlan JM, Wong F and Karsan A (1998) Lipopolysaccharide induces the antiapoptotic molecules, A1 and A20, in microvascular endothelial cells. Blood 92: 2759–2765
Lee HH, Dadgostar H, Cheng Q, Shu J and Cheng G (1999) NF-kappaB-mediated up-regulation of Bcl-x and Bfl-1/A1 is required for CD40 survival signaling in B lymphocytes. Proc. Natl. Acad. Sci. USA 96: 9136–9141
Wang CY, Guttridge DC, Mayo MW and Baldwin Jr AS (1999) NF-kappaB induces expression of the Bcl-2 homologue A1/Bfl-1 to preferentially suppress chemotherapy-induced apoptosis. Mol. Cell. Biol. 19: 5923–5929
Grumont RJ, Rourke IJ and Gerondakis S (1999) Rel-dependent induction of A1 transcription is required to protect B cells from antigen receptor ligation-induced apoptosis. Genes Dev. 13: 400–411 (a) Edelstein LC, Lagos L, Simmons M, Tirumalai H and Gelinas C (2003) NF-κB-dependent assembly of an enhanceosome-like complex on the promoter region of apoptosis inhibitor Bf1-1/A1, Mol. Cell. Biol. 23: 2749–2761
Somogyi RD, Wu Y, Orlofsky A and Prystowsky MB (2001) Transient expression of the Bcl-2 family member, A1-a, results in nuclear localization and resistance to staurosporine-induced apoptosis. Cell Death Diff. 8: 785–793
Werner AB, de Vries E, Tait SWG, Bontjer I and Borst J (2002) Bcl-2 family member Bfl-1/A1 sequesters truncated Bid to inhibit its collaboration with pro-apoptotic Bak or Bax. J. Biol. Chem. 277: 22781–22788
D’Sa-Eipper C, Subramanian T and Chinnadurai G (1996) Bfl-1, a Bcl-2 homologue, suppresses p53-induced apoptosis and exhibits potent cooperative transforming activity. Cancer Res. 56: 3879–3882
Karsan A, Yee E and Harlan JM (1996) Endothelial cell death induced by tumor necrosis factor-alpha is inhibited by the Bcl-2 family member, A1. J. Biol. Chem. 271: 27201–27204
Liang Y, Nylander KD, Yan C and Schor NF (2002) Role of caspase-3-dependent Bcl-2 cleavage in potentiation of apoptosis by Bcl-2. Mol. Pharmacol. 61: 142–149
Kirsch DG, Doseff A, Chau BN, Lim DS, de Souza-Pinto NC, Hansford R, Kastan MB, Lazebnik YA and Hardwick JM (1999) Caspase-3-dependent cleavage of Bcl-2 promotes release of cytochrome c. J. Biol. Chem. 274: 21155–21161
Johnson BW and Boise LH (1999) Bcl-2 and caspase inhibition cooperate to inhibit tumor necrosis factor-alpha-induced cell death in a Bcl-2 cleavage-independent fashion. J. Biol. Chem. 274: 18552–18558
Rock KL, Gramm C, Rothstein L, Clark K, Stein R, Dick L, Hwang D and Goldberg AL (1994) Inhibitors of the proteasome block the degradation of most cell proteins and the generation of peptides presented on MHC class I molecules. Cell 78: 761–771
Fuchs SY, Fried VA and Ronai Z (1998) Stress-activated kinases regulate protein stability. Oncogene 17: 1483–1490
Mandic A, Viktorsson K, Strandberg L, Heiden T, Hansson J, Linder S and Shoshan MC (2002) Calpain-mediated Bid cleavage and calpain-independent Bak modulation: two separate pathways in cisplatin-induced apoptosis. Mol. Cell. Biol. 22: 3003–3013
Wood DE, Thomas A, Devi LA, Berman Y, Beavis RC, Reed JC and Newcomb EW (1998) Bax cleavage is mediated by calpain during drug-induced apoptosis. Oncogene 17: 1069–1078
Choi WS, Lee EH, Chung CW, Jung YK, Jin BK, Kim SU, Oh TH, Saido TC and Oh YJ (2001) Cleavage of Bax is mediated by caspase-dependent or -independent calpain activation in dopaminergic neuronal cells: protective role of Bcl-2. J. Neurochem. 77: 1531–1541
Mellgren RL (1997) Specificities of cell permeant peptidyl inhibitors for the proteinase activities of μ-calpain and the 20S proteasome. J. Biol. Chem. 272: 29899–29903
Griscavage JM, Wilk S and Ignarro LJ (1995) Serine and cysteine proteinase inhibitors prevent nitric oxide production by activated macrophages by interfering with transcription of the inducible NO synthase gene. Biochem. Biophys. Res. Commun. 215: 721–729
Duriez PJ, Wong F, Dorovini-Zis K, Shahidi R and Karsan A (2000) A1 functions at the mitochondria to delay endothelial apoptosis in response to tumor necrosis factor. J. Biol. Chem. 275: 18099–18107
Ko JK, Choi KH, Kim HJ, Choi HY, Yeo DJ, Park SO, Yang WS, Kim YN and Kim CW (2003) Conversion of Bfl-1, an anti-apoptotic Bcl-2 family protein, to a potent pro-apoptotic protein by fusion with green fluorescent protein (GFP). FEBS Lett. 551: 29–36
Cuconati A, Mukherjee C, Perez D and White E (2003) DNA damage response and MCL-1 destruction initiate apoptosis in adenovirus-infected cells. Genes Dev. 17: 2922–2932
Nijhawan D, Fang M, Traer E, Zhong Q, Gao W, Du F and Wang X (2003) Elimination of Mcl-1 is required for the initiation of apoptosis following ultraviolet irradiation. Genes Dev. 17: 1475–1486
Dimmeler S, Breitschopf K, Haendeler J and Zeiher AM (1999) Dephosphorylation targets Bcl-2 for ubiquitin-dependent degradation: a link between the apoptosome and the proteasome pathway. J. Exp. Med. 189: 1815–1822
Breitschopf K, Haendeler J, Malchow P, Zeiher AM and Dimmeler S (2000) Posttranslational modification of Bcl-2 facilitates its proteasome-dependent degradation: molecular characterization of the involved signaling pathway. Mol. Cell. Biol. 20: 1886–1896
Deng X, Gao F, Flagg T and May WS (2004) Mono- and multisite phosphorylation enhances Bcl-2’s antiapoptotic function and inhibition of cell cycle entry functions. Proc. Natl. Acad. Sci. USA 101: 153–158
Goll DE, Thompson VF, Li H, Wei W and Cong J (2003) The calpain system. Physiol. Rev. 83: 731–801
Ruiz-Vela A, Gonzalez de Buitrago G and Martinez AC (1999) Implication of calpain in caspase activation during B cell clonal deletion. EMBO J. 18: 4988–4998
Wolf BB, Goldstein JC, Beere H, Amarante-Mentes GP, Salvesen GS and Green DR (1999) Calpain functions in a caspase-independent manner to promote apoptosis-like events during platelet activation. Blood 94: 1683–1692
Chua BT, Guo K and Li P (2000) Direct cleavage by the calcium-activated protease calpain can lead to inactivation of caspases. J. Biol. Chem. 275: 5131–5135
McGinnis KM, Gnegy ME, Park YH, Mukerjee N and Wang KK (1999) Procaspase-3 and poly(ADP)ribose polymerase (PARP) are calpain substrates. Biochem. Biophys. Res. Commun. 263: 94–99
Porn-Ares MI, Samali A and Orrenius S (1998) Cleavage of the calpain inhibitor, calpastatin, during apoptosis. Cell Death Differ. 5: 1028–1033
Wang KK, Posmantur R, Nadimpalli R, Nath R, Mohan P, Nixon RA, Talanian RV, Keegan M, Herzog L and Allen H (1998) Caspase-mediated fragmentation of calpain inhibitor protein calpastatin during apoptosis. Arch. Biochem. Biophys. 356: 187–196
Saido TC, Sorimachi H and Suzuki K (1994) Calpain: new perspectives in molecular diversity and physiological-pathological involvement. FASEB J. 8: 814–822
Altznauer F, Conus S, Cavalli A, Folkers G and Simon HU (2004) Calpain-1 regulates Bax and subsequent Smac-dependent caspase-3 activation in neutrophil apoptosis. J. Biol. Chem. 279: 5947–5957
Merino R, Ding L, Veis DJ, Korsmeyer SJ and Nunez G (1994) Developmental regulation of the Bcl-2 protein and susceptibility to cell death in B lymphocytes. EMBO J. 13: 683–691
Reed JC (1996) A day in the life of the Bcl-2 protein: does the turnover rate of Bcl-2 serve as a biological clock for cellular lifespan regulation? Leuk. Res. 20: 109–111
Fujita N, Nagahashi A, Nagashima K, Rokudai S and Tsuruo T (1998) Acceleration of apoptotic cell death after cleavage of Bcl-xL protein by caspase-3-like proteases. Oncogene 17: 1295–1304
Chakrabarti AK, Dasgupta S, Gadsden RHS, Hogan EL and Banik NL (1996) Regulation of brain m-calpain Ca2+ sensitivity by mixtures of mambrane lipids: activation at intracellular Ca2+ level. J. Neurosci. Res. 44: 374–380
Melloni E, Pontremoli S, Michetti M, Sacco O, Sparatore B and Horecker BL (1986) The involvement of calpain in the activation of protein kinase C in neutrophils stimulated by phorbol myrstic acid. J. Biol. Chem. 261: 4101–4105
Eto A, Akita Y, Saido TC, Suzuki K and Kawashima S (1995) The role of the calpain–calpastatin system in thyrotropin-releasing hormone-induced selective down-regulation of a protein kinase C isozyme, nPKC epsilon, in rat pituitary GH4C1 cells. J. Biol. Chem. 270: 25115–25120
Alizadeh A, Eisen M, Davis R, Ma C, Lossos I, Rosenwald A, Boldrick JC, Sabet H, Tran T, Yu X, Powell JI, Yang L, Marti GE, Moore T, Hudson Jr J, Lu L, Lewis DB, Tibshirani R, Sherlock G, Chan WC, Greiner TC, Weisenburger DD, Armitage JO, Warnke R, Levy R, Wilson WH, Greyer MR, Byrd JC, Botstein D, Brown PO and Staudt LM (2000) Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature 403: 503–511
Debatin KM, Poncet D and Kroemer G (2002) Chemotherapy: targeting the mitochondrial cell death pathway. Oncogene 21: 8786–8803
Hinz M, Loser P, Mathas S, Krappmann D, Dorken B and Scheidereit C (2001) Constitutive NF-kappaB maintains high expression of a characteristic gene network, including CD40, CD86, and a set of antiapoptotic genes in Hodgkin/Reed–Sternberg cells. Blood 97: 2798–2807
Boise L, Gonzalez-Garcia M, Postema C, Ding L, Lindsten T, Turka L, Mao X, Nunez G and Thompson C (1993) bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell 74: 597–608
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal. Biochem. 72: 248–254
Shumway SD, Maki M and Miyamoto S (1999) The PEST domain of IkBa is necessary and sufficient for in vitro degradation by μ-calpain. J. Biol. Chem. 274: 30874–30881
Acknowledgements
We thank C Labrie, J-Y Springael, E White and R Sundararajan for the gifts of cells and reagents. We are very grateful to E White, R Sundararajan and members of the Gélinas laboratory for discussion and suggestions during the course of this work, to C Gauthier-Rouvière for allowing some experiments to be carried out in her laboratory, and to Petra Pham and Hyejeong Choi for help with FACS analysis. We also thank E White, Y Fan, J Dutta, N Gupta and G Fan for helpful comments on the manuscript. This work was supported by Public Health Service Grant CA83937 from the National Cancer Institute to CG. We acknowledge partial support from the Flow Cytometry Core Facility of the Cancer Institute of New Jersey. JFK was a postdoctoral fellow of the New Jersey Commission on Cancer Research and The Foundation of the UMDNJ. MJS was partially supported by NIH predoctoral training grant in Biochemistry and Molecular Biology GM08360.
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by ME Peter
Rights and permissions
About this article
Cite this article
Kucharczak, J., Simmons, M., Duckett, C. et al. Constitutive proteasome-mediated turnover of Bfl-1/A1 and its processing in response to TNF receptor activation in FL5.12 pro-B cells convert it into a prodeath factor. Cell Death Differ 12, 1225–1239 (2005). https://doi.org/10.1038/sj.cdd.4401684
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/sj.cdd.4401684
Keywords
This article is cited by
-
BCL-2 protein family: attractive targets for cancer therapy
Apoptosis (2023)
-
What can we learn from mice lacking pro-survival BCL-2 proteins to advance BH3 mimetic drugs for cancer therapy?
Cell Death & Differentiation (2022)
-
Characterization of a novel human BFL-1-specific monoclonal antibody
Cell Death & Differentiation (2020)
-
TRIM17 and TRIM28 antagonistically regulate the ubiquitination and anti-apoptotic activity of BCL2A1
Cell Death & Differentiation (2019)
-
BFL1 modulates apoptosis at the membrane level through a bifunctional and multimodal mechanism showing key differences with BCLXL
Cell Death & Differentiation (2019)