Abstract
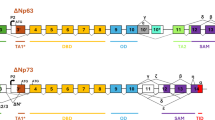
We investigated the mechanisms by which TAp73β and dominant-negative p73 (ΔNp73) regulate apoptosis. TAp73β transactivated the CD95 gene via the p53-binding site in the first intron. In addition, TAp73β induced expression of proapoptotic Bcl-2 family members and led to apoptosis via the mitochondrial pathway. Endogenous TAp73 was upregulated in response to DNA damage by chemotherapeutic drugs. On the contrary, ΔNp73 conferred resistance to chemotherapy. Inhibition of CD95 gene transactivation was one mechanism by which ΔNp73 functionally inactivated the tumor suppressor action of p53 and TAp73β. Concomitantly, ΔNp73 inhibited apoptosis emanating from mitochondria. Thus, ΔNp73 expression in tumors selects against both the death receptor and the mitochondrial apoptosis activity of TAp73β. The importance of these data is evidenced by our finding that upregulation of ΔNp73 in hepatocellular carcinoma patients correlates with reduced survival. Our data indicate that ΔNp73 is an important gene in hepatocarcinogenesis and a relevant prognostic factor.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- ANOVA:
-
analysis of variance
- ΔNp73:
-
dominant-negative p73
- FACS:
-
fluorescence-activated cell sorting
- FADD:
-
Fas-associated death domain
- GFP:
-
green fluorescent protein
- HCC:
-
hepatocellular carcinoma
- MANOVA:
-
multivariate analysis of variance
- MEF:
-
mouse embryo fibroblast
- p53-IBS:
-
intronic p53-binding site
- SAM:
-
significance analysis of microarrays
References
Vousden KH and Lu X (2002) Live or let die: the cell's response to p53. Nat. Rev. Cancer 2: 594–604
Melino G, De Laurenzi V and Vousden KH (2002) p73: friend or foe in tumorigenesis. Nat. Rev. Cancer 2: 605–615
Moll UM and Slade N (2004) p63 and p73: roles in development and tumor formation. Mol. Cancer Res. 2: 371–386
Ramadan S, Terrinoni A, Catani MV, Sayan AE, Knight RA, Mueller M, Krammer PH, Melino G and Candi E (2005) p73 induces apoptosis by different mechanisms. Biochem. Biophys. Res. Commun. 331: 713–717
Yang A, Walker N, Bronson R, Kaghad M, Oosterwegel M, Bonnin J, Vagner C, Bonnet H, Dikkes P, Sharpe A, McKeon F and Caput D (2000) p73-deficient mice have neurological, pheromonal and inflammatory defects but lack spontaneous tumours. Nature 404: 99–103
Kaghad M, Bonnet H, Yang A, Creancier L, Biscan JC, Valent A, Minty A, Chalon P, Lelias JM, Dumont X, Ferrara P, McKeon F and Caput D (1997) Monoallelically expressed gene related to p53 at 1p36, a region frequently deleted in neuroblastoma and other human cancers. Cell 90: 809–819
De Laurenzi V, Costanzo A, Barcaroli D, Terrinoni A and Falco M (1998) Two new p73 splice variants, gamma and delta, with different transcriptional activities. J. Exp. Med. 188: 1768
Zaika AI, Slade N, Erster SH, Sansome C, Joseph TW, Pearl M, Chalas E and Moll UM (2002) DeltaNp73, a dominant-negative inhibitor of wild-type p53 and TAp73, is up-regulated in human tumors. J. Exp. Med. 196: 765–780
Casciano I, Mazzocco K, Boni L, Pagnan G, Banelli B, Allemanni G, Ponzoni M, Tonini GP and Romani M (2002) Expression of DeltaNp73 is a molecular marker for adverse outcome in neuroblastoma patients. Cell Death Differ. 9: 246–251
Petrenko O, Zaika A and Moll UM (2003) DNp73 facilitates cell immortalization and cooperates with oncogenic Ras in cellular transformation in vivo. Mol. Cell. Biol. 23: 5540–5555
Flores ER, Sengupta S, Miller JB, Newman JJ, Bronson R, Crowley D, Yang A, McKeon F and Jacks T (2005) Tumor predisposition in mice mutant for p63 and p73: evidence for broader tumor suppressor functions for the p53 family. Cancer Cell 7: 363–373
Agami R, Blandino G, Oren M and Shaul Y (1999) Interaction of c-Abl and p73alpha and their collaboration to induce apoptosis. Nature 399: 809
Gong JG, Costanzo A, Yang HQ, Melino G, Kaelin Jr WG, Levrero M and Wang JY (1999) The tyrosine kinase c-Abl regulates p73 in apoptotic response to cisplatin-induced DNA damage. Nature 399: 806–809
Gressner O, Schilling T, Lorenz K, Schulze SE, Koch A, Schulze-Bergkamen H, Maria LA, Candi E, Terrinoni A, Valeria CM, Oren M, Melino G, Krammer PH, Stremmel W and Müller M (2005) TAp63alpha induces apoptosis by activating signaling via death receptors and mitochondria. EMBO J. 24: 2458–2471
Flores ER, Tsai KY, Crowley D, Sengupta S, Yang A, McKeon F and Jacks T (2002) p63 and p73 are required for p53-dependent apoptosis in response to DNA damage. Nature 416: 560–564
Senoo M, Manis JP, Alt FW and McKeon F (2004) p63 and p73 are not required for the development and p53-dependent apoptosis of T cells. Cancer Cell 6: 85–89
Müller M, Strand S, Hug H, Heinemann EM, Walczak H, Hofmann WJ, Stremmel W, Krammer PH and Galle PR (1997) Drug-induced apoptosis in hepatoma cells is mediated by the CD95 (APO-1/Fas) receptor/ligand system and involves activation of wild-type p53. J. Clin. Invest. 99: 403–413
Müller M, Wilder S, Bannasch D, Israeli D, Lehlbach K, Li-Weber M, Friedman SL, Galle PR, Stremmel W, Oren M and Krammer PH (1998) p53 activates the CD95 (APO-1/Fas) gene in response to DNA damage by anticancer drugs. J. Exp. Med. 188: 2033–2045
Irwin MS, Kondo K, Marin MC, Cheng LS, Hahn WC and Kaelin WG (2003) Chemosensitivity linked to p73 function. Cancer Cell 3: 403–410
Nicoletti I, Migliorati G, Paggliacci MC, Grignani F and Riccardi C (1991) A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J. Immunol. Methods 139: 271–279
Eichhorst ST, Müller M, Li-Weber M, Schulze-Bergkamen H, Angel P and Krammer PH (2000) A novel AP-1 element in the CD95 ligand promoter is required for induction of apoptosis in hepatocellular carcinoma cells upon treatment with anticancer drugs. Mol. Cell. Biol. 20: 7826–7837
Wu GS, Burns TF, McDonald ER, Jiang W, Meng R, Krantz ID, Kao G, Gan DD, Zhou JY, Muschel R, Hamilton SR, Spinner NB, Markowitz S, Wu G and El-Deiry WS (1997) KILLER/DR5 is a DNA damage-inducible p53-regulated death receptor gene [letter]. Nat. Genet. 17: 141–143
Liu X, Yue P, Khuri FR and Sun SY (2004) p53 upregulates death receptor 4 expression through an intronic p53 binding site. Cancer Res. 64: 5078–5083
Chau BN, Chen TT, Yisong Y, DeGregori J and Wang JYJ (2004) Tumor necrosis factor alpha-induced apoptosis requires p73 and c-ABL activation downstream of RB degradation. Mol. Cell. Biol. 24: 4438–4447
Micheau O, Solary E, Hammann A and Dimanche-Boitrel MT (1999) Fas ligand-independent, FADD-mediated activation of the Fas death pathway by anticancer drugs. J. Biol. Chem. 274: 7987–7992
Boldin MP, Mett IL, Varfolomeev EE, Chumakov I, Shemer-Avni Y, Camonis JH and Wallach D (1995) Self-association of the ‘death domains’ of the p55 tumor necrosis factor (TNF) receptor and Fas/APO1 prompts signaling for TNF and Fas/APO1 effects. J. Biol. Chem. 270: 387–391
Melino G, Bernassola F, Ranalli M, Yee K, Zong WX, Corazzari M, Knight RA, Green DR, Thompson C and Vousden KH (2003) p73 induces apoptosis via PUMA transactivation and Bax mitochondrial translocation. J. Biol. Chem. 279: 8076–8083
Terrasson J, Allart S, Martin H, Lule J, Haddada H, Caput D and Davrinche C (2005) p73-dependent apoptosis through death receptor: impairment by human cytomegalovirus infection. Cancer Res. 65: 2787–2794
Tannapfel A, Wasner M, Krause K, Geissler F, Katalinic A, Hauss J, Mossner J, Engeland K and Wittekind C (1999) Expression of p73 and its relation to histopathology and prognosis in hepatocellular carcinoma. J. Natl. Cancer Inst. 91: 1154–1158
Sayan AE, Paradisi A, Vojtesek B, Knight RA, Melino G and Candi E (2005) New antibodies recognizing p73: comparison with commercial antibodies. Biochem. Biophys. Res. Commun. 330: 186–193
He TC, Zhou S, da Costa LT, Yu J, Kinzler KW and Vogelstein B (1998) A simplified system for generating recombinant adenoviruses. Proc. Natl. Acad. Sci. USA 95: 2509–2514
Miyashita T and Reed JC (1995) Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell 80: 293–299
De Laurenzi V, Raschella G, Barcaroli D, Annicchiarico-Petruzzelli M, Ranalli M, Catani MV, Tanno B, Costanzo A, Levrero M and Melino G (2000) Induction of neuronal differentiation by p73 in a neuroblastoma cell line. J. Biol. Chem. 275: 15226–15231
Tusher VG, Tibshirani R and Chu G (2001) Significance analysis of microarrays applied to the ionizing radiation response. Proc. Natl. Acad. Sci. USA 98: 5116–5121
Hamilton SR and Aaltonen LA (2000) WHO: Pathology and Genetics (Lyon: IARC Press)
Sobin LH and Wittekind CH (2002) UICC: TNM Classification of Malignant Tumours 6th edn (New York: Wiley-Liss)
Tannapfel A, Anhalt K, Hausermann P, Sommerer F, Benicke M, Uhlmann D, Witzigmann H, Hauss J and Wittekind CH (2003) Identification of novel proteins associated with hepatocellular carcinomas using protein microarrays. J. Pathol. 201: 238–249
Wada N, Matsumura M, Ohba Y, Kobayashi N, Takizawa T and Nakanishi Y (1995) Transcription stimulation of the Fas-encoding gene by nuclear factor for interleukin-6 expression upon influenza virus infection. J. Biol. Chem. 270: 18007–18012
El-Deiry WS, Kern SE, Pietenpol JA, Kinzler KW and Vogelstein B (1992) Definition of a consensus binding site for p53. Nat. Genet. 1: 45–49
Brazma A, Hingamp P, Quackenbush J, Sherlock G, Spellman P, Stoeckert C, Aach J, Ansorge W, Ball CA, Causton HC, Gaasterland T, Glenisson P, Holstege FC, Kim IF, Markowitz V, Matese JC, Parkinson H, Robinson A, Sarkans U, Schulze-Kremer S, Stewart J, Taylor R, Vilo J and Vingron M (2001) Minimum information about a microarray experiment (MIAME)-toward standards for microarray data. Nat. Genet. 29: 365–371
Acknowledgements
We thank Petra Hill for expert technical assistance. This work was supported by grants of the Medizinische Forschungsförderung Heidelberg, of the Sonderforschungsbereich 601 and of the Tumorzentrum Heidelberg/Mannheim to MM and PHK. The work was in part performed thanks to grants from AIRC, EU (QLG1-1999-00739 and YLK-CT-2002-01956), MIUR, MinSan to GM, EU (QLK3-CT-2002-01956) to GM and MO and EU grant QLG1-1999-00739 to PHK.
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by D Green
These authors contributed equally to this work
These authors share last authorship
Rights and permissions
About this article
Cite this article
Müller, M., Schilling, T., Sayan, A. et al. TAp73/ΔNp73 influences apoptotic response, chemosensitivity and prognosis in hepatocellular carcinoma. Cell Death Differ 12, 1564–1577 (2005). https://doi.org/10.1038/sj.cdd.4401774
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/sj.cdd.4401774
Keywords
This article is cited by
-
TP73 Isoform-specific disruption reveals a critical role of TAp73beta in growth suppression and inflammatory response
Cell Death & Disease (2023)
-
RNA splicing: a dual-edged sword for hepatocellular carcinoma
Medical Oncology (2022)
-
Overexpression of splicing factor poly(rC)-binding protein 1 elicits cycle arrest, apoptosis induction, and p73 splicing in human cervical carcinoma cells
Journal of Cancer Research and Clinical Oncology (2022)
-
Neutralization of CD95 ligand protects the liver against ischemia-reperfusion injury and prevents acute liver failure
Cell Death & Disease (2018)
-
Improvement of gemcitabine sensitivity of p53-mutated pancreatic cancer MiaPaCa-2 cells by RUNX2 depletion-mediated augmentation of TAp73-dependent cell death
Oncogenesis (2016)