Abstract
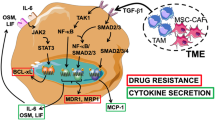
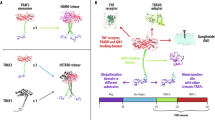
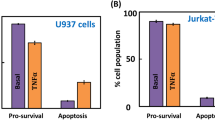
Somatostatin is a multifunctional hormone that modulates cell proliferation, differentiation and apoptosis. Mechanisms for somatostatin-induced apoptosis are at present mostly unsolved. Therefore, we investigated whether somatostatin receptor subtype 2 (sst2) induces apoptosis in the nontransformed murine fibroblastic NIH3T3 cells. Somatostatin receptor subtype 2 expression induced an executioner caspase-mediated apoptosis through a tyrosine phosphatase SHP-1 (Src homology domain phosphatase-1)-dependent stimulation of nuclear factor kappa B (NF-κB) activity and subsequent inhibition of the mitogen-activated protein kinase JNK. Tumor necrosis factor α (TNFα) stimulated both NF-κB and c-Jun NH2-terminal kinase (JNK) activities, which had opposite action on cell survival. Importantly, sst2 sensitized NIH3T3 cells to TNFα-induced apoptosis by (1) upregulating TNFα receptor protein expression, and sensitizing to TNFα-induced caspase-8 activation; (2) enhancing TNFα-mediated activation of NF-κB, resulting in JNK inhibition and subsequent executioner caspase activation and cell death. We have here unraveled a novel signaling mechanism for a G protein-coupled receptor, which directly triggers apoptosis and crosstalks with a death receptor to enhance death ligand-induced apoptosis.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- EMSA:
-
electrophoretic mobility shift assay
- GPCR:
-
G protein-coupled receptor
- IL-1:
-
interleukin-1
- IκB:
-
inhibitory factor-kappa B
- JNK:
-
c-Jun NH2-terminal kinase
- MTT:
-
3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide
- NF-κB:
-
nuclear factor-kappa B
- PAF-R:
-
platelet-activating factor-receptor
- PARP:
-
poly(ADP ribose) polymerase
- SHP-1:
-
Src homology domain phosphatase-1
- sst1–5:
-
somatostatin receptor subtypes 1–5
- TNF:
-
tumor necrosis factor
- TNFR1:
-
tumor necrosis factor receptor 1
- TUNEL:
-
terminal deoxynucleotidyltransferase (TdT)-mediated dUTP-biotin nick end labeling
References
Weckbecker G, Lewis I, Albert R, Schmid HA, Hoyer D, Bruns C . Opportunities in somatostatin research: biological, chemical and therapeutic aspects. Nat Rev Drug Discov 2003; 2: 999–1017.
Bousquet C, Guillermet J, Vernejoul F, Lahlou H, Buscail L, Susini C . Somatostatin receptors and regulation of cell proliferation. Dig Liver Dis 2004; 36 (Suppl 1): S2–S7.
Teijeiro R, Rios R, Costoya JA, Castro R, Bello JL, Devesa J et al. Activation of human somatostatin receptor 2 promotes apoptosis through a mechanism that is independent from induction of p53. Cell Physiol Biochem 2002; 12: 31–38.
Thompson JS . Somatostatin analogue predisposes enterocytes to apoptosis. J Gastrointest Surg 1998; 2: 167–173.
Sharma K, Patel YC, Srikant CB . Subtype-selective induction of wild-type p53 and apoptosis, but not cell cycle arrest, by human somatostatin receptor 3. Mol Endocrinol 1996; 10: 1688–1696.
Pasquali D, Vassallo P, Esposito D, Bonavolonta G, Bellastella A, Sinisi AA . Somatostatin receptor gene expression and inhibitory effects of octreotide on primary cultures of orbital fibroblasts from Graves' ophthalmopathy. J Mol Endocrinol 2000; 25: 63–71.
Tompa A, Jakab MG, Major J, Idei M, Bocsi J, Mihalik R et al. The somatostatin analogue peptide TT-232 induces apoptosis and chromosome breakage in cultured human lymphocytes. Mutat Res 2000; 465: 61–68.
Lattuada D, Casnici C, Venuto A, Marelli O . The apoptotic effect of somatostatin analogue SMS 201–995 on human lymphocytes. J Neuroimmunol 2002; 133: 211–216.
Ferone D, Pivonello R, Van Hagen PM, Dalm VA, Lichtenauer-Kaligis EG, Waaijers M et al. Quantitative and functional expression of somatostatin receptor subtypes in human thymocytes. Am J Physiol Endocrinol Metab 2002; 283: E1056–E1066.
Guillermet J, Saint-Laurent N, Rochaix P, Cuvillier O, Levade T, Schally AV et al. Somatostatin receptor subtype 2 sensitizes human pancreatic cancer cells to death ligand-induced apoptosis. Proc Natl Acad Sci USA 2003; 100: 155–160. Epub 2002 Dec 18.
Rauly I, Saint-Laurent N, Delesque N, Buscail L, Esteve JP, Vaysse N et al. Induction of a negative autocrine loop by expression of sst2 somatostatin receptor in NIH 3T3 cells. J Clin Invest 1996; 97: 1874–1883.
Buscail L, Delesque N, Esteve JP, Saint-Laurent N, Prats H, Clerc P et al. Stimulation of tyrosine phosphatase and inhibition of cell proliferation by somatostatin analogues: mediation by human somatostatin receptor subtypes SSTR1 and SSTR2. Proc Natl Acad Sci USA 1994; 91: 2315–2319.
Margolin N, Raybuck SA, Wilson KP, Chen W, Fox T, Gu Y et al. Substrate and inhibitor specificity of interleukin-1 beta-converting enzyme and related caspases. J Biol Chem 1997; 272: 7223–7228.
Tulipano G, Soldi D, Bagnasco M, Culler MD, Taylor JE, Cocchi D et al. Characterization of new selective somatostatin receptor subtype-2 (sst2) antagonists, BIM-23627 and BIM-23454. Effects of BIM-23627 on GH release in anesthetized male rats after short-term high-dose dexamethasone treatment. Endocrinology 2002; 143: 1218–1224.
Gaur U, Aggarwal BB . Regulation of proliferation, survival and apoptosis by members of the TNF superfamily. Biochem Pharmacol 2003; 66: 1403–1408.
Bousquet C, Delesque N, Lopez F, Saint-Laurent N, Esteve JP, Bedecs K et al. sst2 somatostatin receptor mediates negative regulation of insulin receptor signaling through the tyrosine phosphatase SHP-1. J Biol Chem 1998; 273: 7099–7106.
Ferjoux G, Lopez F, Esteve JP, Ferrand A, Vivier E, Vely F et al. Critical role of Src and SHP-2 in sst2 somatostatin receptor-mediated activation of SHP-1 and inhibition of cell proliferation. Mol Biol Cell 2003; 14: 3911–3928. Epub 2003 Jul 11.
Kucharczak J, Simmons MJ, Fan Y, Gelinas C . To be, or not to be: NF-kappaB is the answer – role of Rel/NF-kappaB in the regulation of apoptosis. Oncogene 2003; 22: 8961–8982.
Lyss G, Knorre A, Schmidt TJ, Pahl HL, Merfort I . The anti-inflammatory sesquiterpene lactone helenalin inhibits the transcription factor NF-kappaB by directly targeting p65. J Biol Chem 1998; 273: 33508–33516.
Lin YZ, Yao SY, Veach RA, Torgerson TR, Hawiger J . Inhibition of nuclear translocation of transcription factor NF-kappa B by a synthetic peptide containing a cell membrane-permeable motif and nuclear localization sequence. J Biol Chem 1995; 270: 14255–14258.
Traenckner EB, Pahl HL, Henkel T, Schmidt KN, Wilk S, Baeuerle PA . Phosphorylation of human I kappa B-alpha on serines 32 and 36 controls I kappa B-alpha proteolysis and NF-kappa B activation in response to diverse stimuli. EMBO J 1995; 14: 2876–2883.
Lin A . Activation of the JNK signaling pathway: breaking the brake on apoptosis. Bioessays 2003; 25: 17–24.
Bennett BL, Sasaki DT, Murray BW, O'Leary EC, Sakata ST, Xu W et al. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc Natl Acad Sci USA 2001; 98: 13681–13686.
Thangaraju M, Sharma K, Leber B, Andrews DW, Shen SH, Srikant CB . Regulation of acidification and apoptosis by SHP-1 and Bcl-2. J Biol Chem 1999; 274: 29549–29557.
Boer U, Fennekohl A, Puschel GP . Sensitization by interleukin-6 of rat hepatocytes to tumor necrosis factor alpha-induced apoptosis. J Hepatol 2003; 38: 728–735.
Ravi R, Bedi GC, Engstrom LW, Zeng Q, Mookerjee B, Gelinas C et al. Regulation of death receptor expression and TRAIL/Apo2L-induced apoptosis by NF-kappaB. Nat Cell Biol 2001; 3: 409–416.
Ryan KM, Ernst MK, Rice NR, Vousden KH . Role of NF-kappaB in p53-mediated programmed cell death. Nature 2000; 404: 892–897.
Kasibhatla S, Brunner T, Genestier L, Echeverri F, Mahboubi A, Green DR . DNA damaging agents induce expression of Fas ligand and subsequent apoptosis in T lymphocytes via the activation of NF-kappa B and AP-1. Mol Cell 1998; 1: 543–551.
Dumont A, Hehner SP, Hofmann TG, Ueffing M, Droge W, Schmitz ML . Hydrogen peroxide-induced apoptosis is CD95-independent, requires the release of mitochondria-derived reactive oxygen species and the activation of NF-kappaB. Oncogene 1999; 18: 747–757.
Kitajima I, Soejima Y, Takasaki I, Beppu H, Tokioka T, Maruyama I . Ceramide-induced nuclear translocation of NF-kappaB is a potential mediator of the apoptotic response to TNF-alpha in murine clonal osteoblasts. Bone 1996; 19: 263–270.
Hunot S, Brugg B, Ricard D, Michel PP, Muriel MP, Ruberg M et al. Nuclear translocation of NF-kappaB is increased in dopaminergic neurons of patients with parkinson disease. Proc Natl Acad Sci USA 1997; 94: 7531–7536.
Li T, Southall MD, Yi Q, Pei Y, Lewis D, Al-Hassani M et al. The epidermal platelet-activating factor receptor augments chemotherapy-induced apoptosis in human carcinoma cell lines. J Biol Chem 2003; 278: 16614–16621. Epub 2003 Feb 24.
Khaled AR, Butfiloski EJ, Sobel ES, Schiffenbauer J . Functional consequences of the SHP-1 defect in motheaten viable mice: role of NF-kappa B. Cell Immunol 1998; 185: 49–58.
Massa PT, Wu C . Increased inducible activation of NF-kappaB and responsive genes in astrocytes deficient in the protein tyrosine phosphatase SHP-1. J Interferon Cytokine Res 1998; 18: 499–507.
Chen BC, Yu CC, Lei HC, Chang MS, Hsu MJ, Huang CL et al. Bradykinin B2 receptor mediates NF-κB activation and cyclooxygenase-2 expression via the Ras/Raf-1/ERK pathway in human airway epithelial cells. J Immunol 2004; 173: 5219–5228.
Lahlou H, Saint-Laurent N, Esteve JP, Eychene A, Pradayrol L, Pyronnet S et al. sst2 Somatostatin receptor inhibits cell proliferation through Ras-, Rap1-, and B-Raf-dependent ERK2 activation. J Biol Chem 2003; 278: 39356–39371.
De Smaele E, Zazzeroni F, Papa S, Nguyen DU, Jin R, Jones J et al. Induction of gadd45beta by NF-kappaB downregulates pro-apoptotic JNK signalling. Nature 2001; 414: 308–313.
Reuther-Madrid JY, Kashatus D, Chen S, Li X, Westwick J, Davis RJ et al. The p65/RelA subunit of NF-kappaB suppresses the sustained, antiapoptotic activity of Jun kinase induced by tumor necrosis factor. Mol Cell Biol 2002; 22: 8175–8183.
Tang G, Minemoto Y, Dibling B, Purcell NH, Li Z, Karin M et al. Inhibition of JNK activation through NF-kappaB target genes. Nature 2001; 414: 313–317.
Shi RX, Ong CN, Shen HM . Luteolin sensitizes tumor necrosis factor-alpha-induced apoptosis in human tumor cells. Oncogene 2004; 23: 7712–7721.
Javelaud D, Besancon F . NF-kappa B activation results in rapid inactivation of JNK in TNF alpha-treated Ewing sarcoma cells: a mechanism for the anti-apoptotic effect of NF-kappa B. Oncogene 2001; 20: 4365–4372.
Yuan ZQ, Feldman RI, Sun M, Olashaw NE, Coppola D, Sussman GE et al. Inhibition of JNK by cellular stress- and tumor necrosis factor alpha-induced AKT2 through activation of the NF kappa B pathway in human epithelial Cells. J Biol Chem 2002; 277: 29973–29982. Epub 2002 Jun 4.
Takeba Y, Suzuki N, Takeno M, Asai T, Tsuboi S, Hoshino T et al. Modulation of synovial cell function by somatostatin in patients with rheumatoid arthritis. Arthritis Rheum 1997; 40: 2128–2138.
Sun XM, MacFarlane M, Zhuang J, Wolf BB, Green DR, Cohen GM . Distinct caspase cascades are initiated in receptor-mediated and chemical-induced apoptosis. J Biol Chem 1999; 274: 5053–5060.
Auernhammer CJ, Bousquet C, Melmed S . Autoregulation of pituitary corticotroph SOCS-3 expression: characterization of the murine SOCS-3 promoter. Proc Natl Acad Sci USA 1999; 96: 6964–6969.
Acknowledgements
We are grateful to C Nahmias (ICGM, Paris, France) for providing SHP-1 constructs. This work was supported by grants from the Association pour la Recherche contre le Cancer (3219), Ligue Régionale contre le Cancer (R04045BB et R04044BB) and EC contract QLG3-CT-1999-00908.
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by JA Trapani
Rights and permissions
About this article
Cite this article
Guillermet-Guibert, J., Saint-Laurent, N., Davenne, L. et al. Novel synergistic mechanism for sst2 somatostatin and TNFα receptors to induce apoptosis: crosstalk between NF-κB and JNK pathways. Cell Death Differ 14, 197–208 (2007). https://doi.org/10.1038/sj.cdd.4401939
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/sj.cdd.4401939
Keywords
This article is cited by
-
Somatostatin analogues in treatment-refractory meningioma: a systematic review with meta-analysis of individual patient data
Neurosurgical Review (2022)
-
The effect of co-administration of Lawsonia inermis extract and octreotide on experimental hepatocellular carcinoma
Environmental Health and Preventive Medicine (2015)
-
Defining the Cancer Master Switch
World Journal of Surgery (2011)
-
Somatostatin analogues in the treatment of gastroenteropancreatic neuroendocrine tumours, current aspects and new perspectives
Journal of Experimental & Clinical Cancer Research (2010)
-
Somatostatin and opioid receptors do not regulate proliferation or apoptosis of the human multiple myeloma U266 cells
Journal of Experimental & Clinical Cancer Research (2009)