Abstract
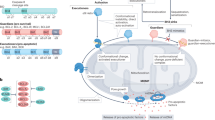
Apoptosis is a phenomenon fundamental to higher eukaryotes and essential to mechanisms controlling tissue homeostasis. Bcl-2 family proteins tightly control this cell death program by regulating the permeabilization of the mitochondrial outer membrane and, hence, the release of cytochrome c and other proapoptotic factors. Mitochondrial apoptosis-induced channel (MAC) is the mitochondrial apoptosis-induced channel and is responsible for cytochrome c release early in apoptosis. MAC activity is detected by patch clamping mitochondria at the time of cytochrome c release. The Bcl-2 family proteins regulate apoptosis by controlling the formation of MAC. Depending on cell type and apoptotic inducer, Bax and/or Bak are structural component(s) of MAC. Overexpression of the antiapoptotic protein Bcl-2 eliminates MAC activity. The focus of this review is a biophysical characterization of MAC activity and its regulation by Bcl-2 family proteins, and ends with some discussion of therapeutic targets.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- PTP:
-
permeability transition pore
- MAC:
-
mitochondrial apoptosis-induced channel
- IL-3:
-
interleukin-3
- VDAC:
-
voltage-dependent anion-selective channel
- ANT:
-
adenine nucleotide translocator
- TOM:
-
translocase of the outer membrane
References
Danial NN and Korsmeyer SJ (2004) Cell death: critical control points. Cell 116: 205–219.
Antonsson B (2004) Mitochondria and the Bcl-2 family proteins in apoptosis signaling pathways. Mol. Cell Biochem. 256–257: 141–155.
Sharpe JC, Arnoult D and Youle RJ (2004) Control of mitochondrial permeability by Bcl-2 family members. Biochim. Biophys. Acta 1644: 107–113.
Green DR and Kroemer G (2004) The pathophysiology of mitochondrial cell death. Science 305: 626–629.
Kuwana T and Newmeyer DD (2003) Bcl-2-family proteins and the role of mitochondria in apoptosis. Curr. Opin. Cell Biol. 15: 691–699.
Fadeel B and Orrenius S (2005) Apoptosis: a basic biological phenomenon with wide-ranging implications in human disease. J. Intern. Med. 258: 479–517.
Lucken-Ardjomande S and Martinou JC (2005) Regulation of Bcl-2 proteins and of the permeability of the outer mitochondrial membrane. C. R. Biol. 328: 616–631.
Martinez-Caballero S, Dejean LM, Jonas EA and Kinnally KW (2005) The role of the mitochondrial apoptosis induced channel MAC in cytochrome c release. J. Bioenerg. Biomembr. 37: 155–164.
Dejean LM, Martinez-Caballero S, Manon S and Kinnally KW (2006) Regulation of the mitochondrial apoptosis-induced channel, MAC, by BCL-2 family proteins. Biochim. Biophys. Acta 1762: 191–201.
Schmitz I, Kirchhoff S and Krammer PH (2000) Regulation of death receptor-mediated apoptosis pathways. Int. J. Biochem. Cell. Biol. 32: 1123–1136.
Liu X, Kim CN, Yang J, Jemmerson R and Wang X (1996) Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell 86: 147–157.
Juin P, Cartron PF and Vallette FM (2005) Activation of Bax by BH3 domains during apoptosis: the unfolding of a deadly plot. Cell Cycle 4: 637–642.
Li H, Zhu H, Xu CJ and Yuan J (1998) Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell 94: 491–501.
Kroemer G and Reed JC (2000) Mitochondrial control of cell death. Nat. Med. 6: 513–519.
Skulachev VP (1996) Why are mitochondria involved in apoptosis? Permeability transition pores and apoptosis as selective mechanisms to eliminate superoxide-producing mitochondria and cell. FEBS Lett. 397: 7–10.
Marzo I, Brenner C, Zamzami N, Susin SA, Beutner G, Brdiczka D, Remy R, Xie ZH, Reed JC and Kroemer G (1998) The permeability transition pore complex: a target for apoptosis regulation by caspases and bcl-2-related proteins. J. Exp. Med. 187: 1261–1271.
Nakagawa T, Shimizu S, Watanabe T, Yamaguchi O, Otsu K, Yamagata H, Inohara H, Kubo T and Tsujimoto Y (2005) Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature 434: 652–658.
Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, Robbins J and Molkentin JD (2005) Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature 434: 658–662.
Basso E, Fante L, Fowlkes J, Petronilli V, Forte MA and Bernardi P (2005) Properties of the permeability transition pore in mitochondria devoid of Cyclophilin D. J. Biol. Chem. 280: 18558–18561.
Pavlov EV, Priault M, Pietkiewicz D, Cheng EH, Antonsson B, Manon S, Korsmeyer SJ, Mannella CA and Kinnally KW (2001) A novel, high conductance channel of mitochondria linked to apoptosis in mammalian cells and Bax expression in yeast. J. Cell. Biol. 155: 725–731.
Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB and Korsmeyer SJ (2001) Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science 292: 727–730.
De Giorgi F, Lartigue L, Bauer MK, Schubert A, Grimm S, Hanson GT, Remington SJ, Youle RJ and Ichas F (2002) The permeability transition pore signals apoptosis by directing Bax translocation and multimerization. FASEB J. 16: 607–609.
Guo L, Pietkiewicz D, Pavlov EV, Grigoriev SM, Kasianowicz JJ, Dejean LM, Korsmeyer SJ, Antonsson B and Kinnally KW (2004) Effects of cytochrome c on the mitochondrial apoptosis-induced channel MAC. Am .J. Physiol. Cell Physiol. 286: C1109–C1117.
Dejean LM, Martinez-Caballero S, Guo L, Hughes C, Teijido O, Ducret T, Ichas F, Korsmeyer SJ, Antonsson B, Jonas EA and Kinnally KW (2005) Oligomeric Bax is a component of the putative cytochrome c release channel MAC, mitochondrial apoptosis-induced channel. Mol. Biol. Cell. 16: 2424–2432.
Scorrano L and Korsmeyer SJ (2003) Mechanisms of cytochrome c release by proapoptotic BCL-2 family members. Biochem. Biophys. Res. Commun. 304: 437–444.
Rostovtseva T and Colombini M (1997) VDAC channels mediate and gate the flow of ATP: implications for the regulation of mitochondrial function. Biophys. J. 72: 1954–1962.
Rostovtseva TK and Bezrukov SM (1998) ATP transport through a single mitochondrial channel, VDAC, studied by current fluctuation analysis. Biophys. J. 74: 2365–2373.
Rostovtseva TK, Komarov A, Bezrukov SM and Colombini M (2002) Dynamics of nucleotides in VDAC channels: structure-specific noise generation. Biophys. J. 82: 193–205.
Martinez-Caballero S, Dejean LM and Kinnally KW (2004) Some amphiphilic cations block the mitochondrial apoptosis-induced channel, MAC. FEBS Lett. 568: 35–38.
Guihard G, Bellot G, Moreau C, Pradal G, Ferry N, Thomy R, Fichet P, Meflah K and Vallette FM (2004) The mitochondrial apoptosis-induced channel (MAC) corresponds to a late apoptotic event. J. Biol. Chem. 279: 46542–46550.
Truscott KN, Kovermann P, Geissler A, Merlin A, Meijer M, Driessen AJ, Rassow J, Pfanner N and Wagner R (2001) A presequence- and voltage-sensitive channel of the mitochondrial preprotein translocase formed by Tim23. Nat. Struct. Biol. 8: 1074–1082.
Krasilnikov OV, Sabirov RZ, Ternovsky VI, Merzliak PG and Muratkhodjaev JN (1992) A simple method for the determination of the pore radius of ion channels in planar lipid bilayer membranes. FEMS Microbiol. Immunol. 5: 93–100.
Krasilnikov OV, Da Cruz JB, Yuldasheva LN, Varanda WA and Nogueira RA (1998) A novel approach to study the geometry of the water lumen of ion channels: colicin Ia channels in planar lipid bilayers. J. Membr. Biol. 161: 83–92.
Grigoriev SM, Muro C, Dejean LM, Campo ML, Martinez-Caballero S and Kinnally KW (2004) Electrophysiological approaches to the study of protein translocation in mitochondria. Int. Rev. Cytol. 238: 227–274.
Bezrukov SM and Kasianowicz JJ (1997) The charge state of an ion channel controls neutral polymer entry into its pore. Eur. Biophys. J. 26: 471–476.
Chan SK, Tulloss I and Margoliash E (1966) Primary structure of the cytochrome c from the snapping turtle, Chelydra serpentina. Biochemistry 5: 2586–2597.
Gupte SS and Hackenbrock CR (1988) The role of cytochrome c diffusion in mitochondrial electron transport. J. Biol. Chem. 263: 5248–5253.
Tang QY, Qi Z, Naruse K and Sokabe M (2003) Characterization of a functionally expressed stretch-activated BKca channel cloned from chick ventricular myocytes. J. Membr. Biol. 196: 185–200.
Moczydlowski E (1986) Single channel enzymology. In Ion Channel Reconstitution, Miller C (ed) (Plenum Press: New York, NY) pp. 75–113.
Akeson M, Branton D, Kasianowicz JJ, Brandin E and Deamer DW (1999) Microsecond time-scale discrimination among polycytidylic acid, polyadenylic acid, and polyuridylic acid as homopolymers or as segments within single RNA molecules. Biophys. J. 77: 3227–3233.
Kullman L, Winterhalter M and Bezrukov SM (2002) Transport of maltodextrins through maltoporin: a single-channel study. Biophys. J. 82: 803–812.
Nestorovich EM, Danelon C, Winterhalter M and Bezrukov SM (2002) Designed to penetrate: time-resolved interaction of single antibiotic molecules with bacterial pores. Proc. Natl. Acad. Sci. USA 99: 9789–9794.
Gross A, Jockel J, Wei MC and Korsmeyer SJ (1998) Enforced dimerization of BAX results in its translocation, mitochondrial dysfunction and apoptosis. EMBO J. 17: 3878–3885.
Muchmore SW, Sattler M, Liang H, Meadows RP, Harlan JE, Yoon HS, Nettesheim D, Chang BS, Thompson CB, Wong SL, Ng SL and Fesik SW (1996) X-ray and NMR structure of human Bcl-xL, an inhibitor of programmed cell death. Nature 381: 335–341.
Schlesinger PH, Gross A, Yin XM, Yamamoto K, Saito M, Waksman G and Korsmeyer SJ (1997) Comparison of the ion channel characteristics of proapoptotic BAX and antiapoptotic BCL-2. Proc. Natl. Acad. Sci. USA 94: 11357–11362.
Antonsson B, Conti F, Ciavatta A, Montessuit S, Lewis S, Martinou I, Bernasconi L, Bernard A, Mermod JJ, Mazzei G, Maundrell K, Gambale F, Sadoul R and Martinou JC (1997) Inhibition of Bax channel-forming activity by Bcl-2. Science 277: 370–372.
Minn AJ, Velez P, Schendel SL, Liang H, Muchmore SW, Fesik SW, Fill M and Thompson CB (1997) Bcl-x(L) forms an ion channel in synthetic lipid membranes. Nature 385: 353–357.
Schendel SL, Azimov R, Pawlowski K, Godzik A, Kagan BL and Reed JC (1999) Ion channel activity of the BH3 only Bcl-2 family member, BID. J. Biol. Chem. 274: 21932–21936.
Schendel SL, Xie Z, Montal MO, Matsuyama S, Montal M and Reed JC (1997) Channel formation by antiapoptotic protein Bcl-2. Proc. Natl. Acad. Sci. USA 94: 5113–5118.
Stroud R (1995) Ion channel forming colicins. Curr. Opin. Struct. Biol. 5: 514–520.
Murphy RC, Schneider E and Kinnally KW (2001) Overexpression of Bcl-2 suppresses the calcium activation of a mitochondrial megachannel. FEBS Lett. 497: 73–76.
Roucou X, Rostovtseva T, Montessuit S, Martinou JC and Antonsson B (2002) Bid induces cytochrome c-impermeable Bax channels in liposomes. Biochem. J. 363: 547–552.
Priault M, Camougrand N, Kinnally KW, Vallette FM and Manon S (2003) Yeast as a tool to study Bax/mitochondrial interactions in cell death. FEMS Yeast. Res. 4: 15–27.
Hanada M, Aime-Sempe C, Sato T and Reed JC (1995) Structure-function analysis of Bcl-2 protein. Identification of conserved domains important for homodimerization with Bcl-2 and heterodimerization with Bax. J. Biol. Chem. 270: 11962–11969.
Manon S, Chaudhuri B and Guerin M (1997) Release of cytochrome c and decrease of cytochrome c oxidase in Bax-expressing yeast cells, and prevention of these effects by coexpression of Bcl-xL. FEBS Lett. 415: 29–32.
Antonsson B, Montessuit S, Sanchez B and Martinou JC (2001) Bax is present as a high molecular weight oligomer/complex in the mitochondrial membrane of apoptotic cells. J. Biol. Chem. 276: 11615–11623.
Mikhailov V, Mikhailov M, Pulkrabek DJ, Dong Z, Venkatachalam MA and Saikumar P (2001) Bcl-2 prevents bax oligomerization in the mitochondrial outer membrane. J. Biol. Chem. 276: 18361–18374.
Nechushtan A, Smith CL, Hsu YT and Youle RJ (1999) Conformation of the Bax C-terminus regulates subcellular location and cell death. EMBO J. 18: 2330–2341.
Desagher S, Osen-Sand A, Nichols A, Eskes R, Montessuit S, Lauper S, Maundrell K, Antonsson B and Martinou JC (1999) Bid-induced conformational change of Bax is responsible for mitochondrial cytochrome c release during apoptosis. J. Cell. Biol. 144: 891–901.
Wei MC, Lindsten T, Mootha VK, Weiler S, Gross A, Ashiya M, Thompson CB and Korsmeyer SJ (2000) tBID, a membrane-targeted death ligand, oligomerizes BAK to release cytochrome c. Genes Dev. 14: 2060–2071.
Eskes R, Desagher S, Antonsson B and Martinou JC (2000) Bid induces the oligomerization and insertion of Bax into the outer mitochondrial membrane. Mol. Cell. Biol. 20: 929–935.
Jonas EA, Hickman JA, Chachar M, Polster BM, Brandt TA, Fannjiang Y, Ivanovska I, Basanez G, Kinnally KW, Zimmerberg J, Hardwick JM and Kaczmarek LK (2004) Proapoptotic N-truncated BCL-xL protein activates endogenous mitochondrial channels in living synaptic terminals. Proc. Natl. Acad. Sci. USA 101: 13590–13595.
Jonas EA, Hickman JA, Hardwick JM and Kaczmarek LK (2005) Exposure to hypoxia rapidly induces mitochondrial channel activity within a living synapse. J. Biol. Chem. 280: 4491–4497.
Lenartowicz E, Bernardi P and Azzone GF (1991) Phenylarsine oxide induces the cyclosporin A-sensitive membrane permeability transition in rat liver mitochondria. J. Bioenerg. Biomembr. 23: 679–688.
Szabo I, Bernardi P and Zoratti M (1992) Modulation of the mitochondrial megachannel by divalent cations and protons. J. Biol. Chem. 267: 2940–2946.
Broekemeier KM, Carpenter-Deyo L, Reed DJ and Pfeiffer DR (1992) Cyclosporin A protects hepatocytes subjected to high Ca2+ and oxidative stress. FEBS Lett. 304: 192–194.
Nieminen AL, Saylor AK, Tesfai SA, Herman B and Lemasters JJ (1995) Contribution of the mitochondrial permeability transition to lethal injury after exposure of hepatocytes to t-butylhydroperoxide. Biochem. J. 307 (Part 1): 99–106.
Freedman AM, Kramer JH, Mak IT, Cassidy MM and Weglicki WB (1991) Propranolol preserves ultrastructure in adult cardiocytes exposed to anoxia/reoxygenation: a morphometric analysis. Free Radic. Biol. Med. 11: 197–206.
Hoyt KR, Sharma TA and Reynolds IJ (1997) Trifluoperazine and dibucaine-induced inhibition of glutamate-induced mitochondrial depolarization in rat cultured forebrain neurones. Br. J. Pharmacol. 122: 803–808.
Polster BM, Basanez G, Young M, Suzuki M and Fiskum G (2003) Inhibition of Bax-induced cytochrome c release from neural cell and brain mitochondria by dibucaine and propranolol. J. Neurosci. 23: 2735–2743.
Bombrun A, Gerber P, Casi G, Terradillos O, Antonsson B and Halazy S (2003) 3, 6-dibromocarbazole piperazine derivatives of 2-propanol as first inhibitors of cytochrome c release via Bax channel modulation. J. Med. Chem. 46: 4365–4368.
Hetz C, Vitte PA, Bombrun A, Rostovtseva TK, Montessuit S, Hiver A, Schwarz MK, Church DJ, Korsmeyer SJ, Martinou JC and Antonsson B (2005) Bax channel inhibitors prevent mitochondrion-mediated apoptosis and protect neurons in a model of global brain ischemia. J. Biol. Chem. 280: 42960–42970.
Roucou X, Montessuit S, Antonsson B and Martinou JC (2002) Bax oligomerization in mitochondrial membranes requires tBid (caspase-8-cleaved Bid) and a mitochondrial protein. Biochem. J. 368: 915–921.
Jensen RE (2005) Control of mitochondrial shape. Curr. Opin. Cell. Biol. 17: 384–388.
Youle RJ and Karbowski M (2005) Mitochondrial fission in apoptosis. Nat. Rev. Mol. Cell. Biol. 6: 657–663.
Scorrano L (2005) Proteins that fuse and fragment mitochondria in apoptosis: con-fissing a deadly con-fusion? J. Bioenerg. Biomembr. 37: 165–170.
Roesch K, Curran SP, Tranebjaerg L and Koehler CM (2002) Human deafness dystonia syndrome is caused by a defect in assembly of the DDP1/TIMM8a-TIMM13 complex. Hum. Mol. Genet. 11: 477–486.
Koehler CM (2004) New developments in mitochondrial assembly. Annu. Rev. Cell. Dev. Biol. 20: 309–335.
Hille B (2001) Ionic Channels of Excitable Membranes. (Sunderland, MA: Sinauer Assoc.) pp. 351–352.
Acknowledgements
This research was supported by NIH Grant GM57249 and NSF Grants MCB-0235834 and INT003797 to KWK. We thank Stephen Manon (Bordeaux, France) for his insightful and lively discussions and Cynthia Hughes for her excellent technical assistance. Finally, we gratefully acknowledge the huge contribution of Stan Korsmeyer to our own research program through his many years of active support and intellectual stimulation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by C Borner
Rights and permissions
About this article
Cite this article
Dejean, L., Martinez-Caballero, S. & Kinnally, K. Is MAC the knife that cuts cytochrome c from mitochondria during apoptosis?. Cell Death Differ 13, 1387–1395 (2006). https://doi.org/10.1038/sj.cdd.4401949
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/sj.cdd.4401949
Keywords
This article is cited by
-
Cytotoxicity of the methanol extracts and compounds of Brucea antidysenterica (Simaroubaceae) towards multifactorial drug-resistant human cancer cell lines
BMC Complementary Medicine and Therapies (2023)
-
The novel protective role of P27 in MLN4924-treated gastric cancer cells
Cell Death & Disease (2015)
-
Chemical glycosylation of cytochrome c improves physical and chemical protein stability
BMC Biochemistry (2014)
-
Fenvalerate induces germ cell apoptosis in mouse testes through the Fas/FasL signaling pathway
Archives of Toxicology (2011)
-
Mitochondrial matters of the brain: the role in Huntington’s disease
Journal of Bioenergetics and Biomembranes (2010)