Abstract
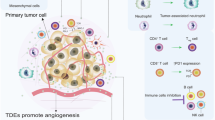
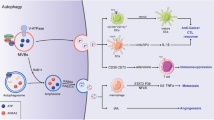
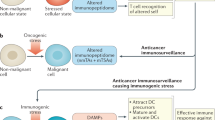
Tumour cells release vesicular structures, defined as microvesicles or exosomes, carrying a large array of proteins from their originating cell. The expression of antigenic molecules recognized by T cells has originally suggested a role for these organelles as a cell-free antigen source for anticancer vaccines. However, recent evidence shows that tumour exosomes may also exert a broad array of detrimental effects on the immune system, ranging from apoptosis in activated antitumour T cells to impairment of monocyte differentiation into dendritic cells and induction of myeloid suppressive cells. Immunosuppressive exosomes of tumour origin can be found in neoplastic lesions and sera from cancer patients, implying a potential role of this pathway in in vivo tumour progression. Through the expression of molecules involved in angiogenesis promotion, stromal remodelling, delivery of signalling pathways through growth factor/receptor transfer, chemoresistance and genetic intercellular exchange, tumour exosomes could represent a versatile tool for moulding host environment. Hence, their secretion by neoplastic cells may in the future become a novel pathway to target for therapeutic intervention in cancer patients.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- APC:
-
antigen-presenting cell
- DC:
-
dendritic cell
- DEX:
-
DC exosome
- ESCRT:
-
endosomal sorting complexes required for transport
- HSP:
-
heat-shock protein
- LN:
-
lymph node
- MMP:
-
matrix metalloproteinase
- MSC:
-
myeloid suppressor cell
- MVB:
-
multivesicular body
- PPI:
-
proton pump inhibitor
References
Balkwill F, Charles KA, Mantovani A . Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell 2005; 7: 211–217.
Lin WW, Karin M . A cytokine-mediated link between innate immunity, inflammation, and cancer. J Clin Invest 2007; 117: 1175–11831.
Rivoltini L, Canese P, Huber V, Iero M, Pilla L, Valenti R et al. Escape strategies and reasons for failure in the interaction between tumour cells and the immune system: how can we tilt the balance towards immune-mediated cancer control? Expert Opin Biol Ther 2005; 5: 463–476.
Houghton AN . Cancer antigens: immune recognition of self and altered self. J Exp Med 1994; 180: 1–4.
Dunn GP, Old LJ, Schreiber RD . The immunobiology of cancer immunosurveillance and immunoediting. Immunity 2004; 21: 137–148.
Zou W . Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer 2005; 5: 263–274.
Hahn WC, Weinberg RA . Rules for making human tumor cells. N Engl J Med 2002; 347: 1593–1603.
Gajewski TF, Meng Y, Blank C, Brown I, Kacha A, Kline J et al. Immune resistance orchestrated by the tumor microenvironment. Immunol Rev 2006; 213: 131–145.
Rabinovich GA, Gabrilovich D, Sotomayor EM . Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol 2007; 25: 267–296.
Gabrilovich D . Mechanisms and functional significance of tumour-induced dendritic-cell defects. Nat Rev Immunol 2004; 4: 941–952.
Do TH, Johnsen HE, Kjaersgaard E, Taaning E, Svane IM . Impaired circulating myeloid DCs from myeloma patients. Cytotherapy 2004; 6: 196–203.
Vakkila J, Thomson AW, Vettenranta K, Sariola H, Saarinen-Pihkala UM . Dendritic cell subsets in childhood and in children with cancer: relation to age and disease prognosis. Clin Exp Immunol 2004; 135: 455–461.
Della Bella S, Gennaro M, Vaccari M, Ferraris C, Nicola S, Riva A et al. Altered maturation of peripheral blood dendritic cells in patients with breast cancer. Br J Cancer 2003; 89: 1463–1472.
Chaux P, Moutet M, Faivre J, Martin F, Martin M . Inflammatory cells infiltrating human colorectal carcinomas express HLA class II but not B7-1 and B7-2 costimulatory molecules of the T-cell activation. Lab Invest 1996; 74: 975–983.
Whiteside TL, Stanson J, Shurin MR, Ferrone S . Antigen-processing machinery in human dendritic cells: up-regulation by maturation and down-regulation by tumor cells. J Immunol 2004; 173: 1526–1534.
Filipazzi P, Valenti R, Huber V, Pilla L, Canese P, Iero M et al. Identification of a new subset of myeloid suppressor cells in peripheral blood of melanoma patients with modulation by a granulocyte–macrophage colony-stimulation factor-based antitumor vaccine. J Clin Oncol 2007; 25: 2546–2553.
Bronte V, Kasic T, Gri G, Gallana K, Borsellino G, Marigo I et al. Boosting antitumor responses of T lymphocytes infiltrating human prostate cancers. J Exp Med 2005; 201: 1257–1268.
Serafini P, Carbley R, Noonan KA, Tan G, Bronte V, Borrello I . High-dose granulocyte-macrophage colony-stimulating factor-producing vaccines impair the immune response through the recruitment of myeloid suppressor cells. Cancer Res 2004; 64: 6337–6343.
Almand B, Resser JR, Lindman B, Nadaf S, Clark JI, Kwon ED et al. Clinical significance of defective dendritic cell differentiation in cancer. Clin Cancer Res 2000; 6: 1755–1766.
Ohm JE, Carbone DP . VEGF as a mediator of tumor-associated immunodeficiency. Immunol Res 2001; 23: 263–272.
Pιguet-Navarro J, Sportouch M, Popa I, Berthier O, Schmitt D, Portoukalian J . Gangliosides from human melanoma tumors impair dendritic cell differentiation from monocytes and induce their apoptosis. J Immunol 2003; 170: 3488–3494.
Gosset P, Bureau F, Angeli V, Pichavant M, Faveeuw C, Tonnel AB et al. Prostaglandin D2 affects the maturation of human monocyte-derived dendritic cells: consequence on the polarization of naive Th cells. J Immunol 2003; 170: 4943–4952.
Brown R, Murray A, Pope B, Sze DM, Gibson J, Ho PJ et al. Either interleukin-12 or interferon-gamma can correct the dendritic cell defect induced by transforming growth factor beta in patients with myeloma. Br J Haematol 2004; 125: 743–748.
van Niel G, Porto-Carreiro I, Simoes S, Raposo G . Exosomes: a common pathway for a specialized function. J Biochem (Tokyo) 2006; 140: 13–21.
Sϕderberg A, Barral AM, Sϕderstrϕm M, Sander B, Rosιn A . Redox-signaling transmitted in trans to neighboring cells by melanoma-derived TNF-containing exosomes. Free Radic Biol Med 2007; 43: 90–99.
Clayton A, Mitchell JP, Court J, Mason MD, Tabi Z . Human tumor-derived exosomes selectively impair lymphocyte responses to interleukin-2. Cancer Res 2007; 67: 7458–7466.
Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D et al. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat Med 1998; 4: 594–600.
Thιry C, Zitvogel L, Amigorena S . Exosomes: composition, biogenesis and function. Nat Rev Immunol 2002; 2: 569–579.
Andre F, Schartz NE, Movassagh M, Flament C, Pautier P, Morice P et al. Malignant effusions and immunogenic tumour-derived exosomes. Lancet 2002; 360: 295–305.
Escudier B, Dorval T, Chaput N, Andre F, Caby MP, Novault S et al. Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: results of the first phase I clinical trial. J Transl Med 2005; 3: 10.
Morse MA, Garst J, Osada T, Khan S, Hobeika A, Clay TM . A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer. J Transl Med 2005; 3: 9.
Huber V, Fais S, Iero M, Lugini L, Canese P, Squarcina P et al. Human colorectal cancer cells induce T-cell death through release of proapoptotic microvesicles: role in immune escape. Gastroenterology 2005; 128: 1796–1804.
Valenti R, Huber V, Filipazzi P, Pilla L, Sovena G, Villa A et al. Human tumor-released microvesicles promote the differentiation of myeloid cells with transforming growth factor-beta-mediated suppressive activity on T lymphocytes. Cancer Res 2006; 66: 9290–9298.
Andreola G, Rivoltini L, Castelli C, Huber V, Perego P, Deho P et al. Induction of lymphocyte apoptosis by tumor cell secretion of FasL-bearing microvesicles. J Exp Med 2002; 195: 1303–1316.
Mears R, Craven RA, Hanrahan S, Totty N, Upton C, Young SL et al. Proteomic analysis of melanoma-derived exosomes by two-dimensional polyacrylamide gel electrophoresis and mass spectrometry. Proteomics 2004; 4: 4019–4031.
Hegmans JP, Bard MP, Hemmes A, Luider TM, Kleijmeer MJ, Prins JB et al. Proteomic analysis of exosomes secreted by human mesothelioma cells. Am J Pathol 2004; 164: 1807–1815.
Srivastava P . Interaction of heat shock proteins with peptides and antigen presenting cells: chaperoning of the innate and adaptive immune responses. Annu Rev Immunol 2002; 20: 395–425.
Wolfers J, Lozier A, Raposo G, Regnault A, Thιry C, Masurier C et al. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat Med 2001; 7: 297–303.
Serafini P, Borrello I, Bronte V . Myeloid suppressor cells in cancer: recruitment, phenotype, properties, and mechanisms of immune suppression. Semin Cancer Biol 2006; 16: 53–65.
Parmiani G, Castelli C, Pilla L, Santinami M, Colombo MP, Rivoltini L . Opposite immune functions of GM-CSF administered as vaccine adjuvant in cancer patients. Ann Oncol 2007; 18: 226–232.
Valenti R, Huber V, Iero M, Filipazzi P, Parmiani G, Rivoltini L . Tumor-released microvesicles as vehicles of immunosuppression. Cancer Res 2007; 67: 2912–2915.
Wieckowski E, Whiteside TL . Human tumor-derived vs dendritic cell-derived exosomes have distinct biologic roles and molecular profiles. Immunol Res 2006; 16: 415–421.
Monleσn I, Martνnez-Lorenzo MJ, Monteagudo L, Lasierra P, Taulιs M, Iturralde M et al. Differential secretion of Fas ligand- or APO2 ligand/TNF-related apoptosis-inducing ligand-carrying microvesicles during activation-induced death of human T cells. J Immunol 2001; 167: 6736–6744.
Sabapatha A, Gercel-Taylor C, Taylor DD . Specific isolation of placenta-derived exosomes from the circulation of pregnant women and their immunoregulatory consequences. Am J Reprod Immunol 2006; 56: 345–355.
Taylor DD, Akyol S, Gercel-Taylor C . Pregnancy-associated exosomes and their modulation of T cell signaling. J Immunol 2006; 176: 1534–1542.
Taylor DD, Gerηel-Taylor C . Tumour-derived exosomes and their role in cancer-associated T-cell signalling defects. Br J Cancer 2005; 92: 305–311.
Kim JW, Wieckowski E, Taylor DD, Reichert TE, Watkins S, Whiteside TL . Fas ligand-positive membranous vesicles isolated from sera of patients with oral cancer induce apoptosis of activated T lymphocytes. Clin Cancer Res 2005; 11: 1010–1020.
Liu C, Yu S, Zinn K, Wang J, Zhang L, Jia Y et al. Murine mammary carcinoma exosomes promote tumor growth by suppression of NK cell function. J Immunol 2006; 176: 1375–1385.
Whiteside TL . Tumour-derived exosomes or microvesicles: another mechanism of tumour escape from the host immune system? Br J Cancer 2005; 92: 209–211.
Yu S, Liu C, Su K, Wang J, Liu Y, Zhang L et al. Tumor exosomes inhibit differentiation of bone marrow dendritic cells. J Immunol 2007; 178: 6867–6875.
Fιvrier B, Raposo G . Exosomes: endosomal-derived vesicles shipping extracellular messages. Curr Opin Cell Biol 2004; 16: 415–421.
Subra C, Laulagnier K, Perret B, Record M . Exosome lipidomics unravels lipid sorting at the level of multivesicular bodies. Biochimie 2007; 89: 205–212.
Denzer K, Kleijmeer MJ, Heijnen HF, Stoorvogel W, Geuze HJ . Exosome: from internal vesicle of the multivesicular body to intercellular signaling device. J Cell Sci 2000; 113: 3365–3374.
Calzolari A, Raggi C, Deaglio S, Sposi NM, Stafsnes M, Fecchi K et al. TfR2 localizes in lipid raft domains and is released in exosomes to activate signal transduction along the MAPK pathway. J Cell Sci 2006; 119: 4486–4498.
Hao S, Ye Z, Li F, Meng Q, Qureshi M, Yang J et al. Epigenetic transfer of metastatic activity by uptake of highly metastatic B16 melanoma cell-released exosomes. Exp Oncol 2006; 28: 126–131.
Gesierich S, Berezovskiy I, Ryschich E, Zϕller M . Systemic induction of the angiogenesis switch by the tetraspanin D61A/CO-029. Cancer Res 2006; 66: 7083–7094.
Valadi H, Ekstrϕm K, Bossios A, Sjϕstrand M, Lee JJ, Lϕtvall JO . Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 2007; 9: 654–659.
Safaei R, Larson BJ, Cheng TC, Gibson MA, Otani S, Naerdemann W et al. Abnormal lysosomal trafficking and enhanced exosomal export of cisplatin in drug-resistant human ovarian carcinoma cells. Mol Cancer Ther 2005; 4: 1595–1604.
Chen KG, Valencia JC, Lai B, Zhang G, Paterson JK, Rouzaud F et al. Melanosomal sequestration of cytotoxic drugs contributes to the intractability of malignant melanomas. Proc Natl Acad Sci USA 2006; 103: 9903–9907.
Kroemer G, Jaattela M . Lysosomes and autophagy in cell death control. Nat Rev Cancer 2005; 5: 886–897.
Shedden K, Xie XT, Chandaroy P, Chang YT, Rosania GR . Expulsion of small molecules in vesicles shed by cancer cells: association with gene expression and chemosensitivity profiles. Cancer Res 2003; 63: 4331–4337.
Yu X, Harris SL, Levine AJ . The regulation of exosome secretion: a novel function of the p53 protein. Cancer Res 2006; 66: 4795–4801.
Groth-Pedersen L, Ostenfeld MS, Hψyer-Hansen M, Nylandsted J, Jδδttelδ M . Vincristine induces dramatic lysosomal changes and sensitizes cancer cells to lysosome-destabilizing siramesine. Cancer Res 2007; 67: 2217–2225.
Liιgeois S, Benedetto A, Garnier JM, Schwab Y, Labouesse M . The V0-ATPase mediates apical secretion of exosomes containing Hedgehog-related proteins in Caenorhabditis elegans. J Cell Biol 2006; 173: 949–961.
Luciani F, Spada M, De Milito A, Molinari A, Rivoltini L, Montinaro A et al. Effect of proton pump inhibitor pretreatment on resistance of solid tumors to cytotoxic drugs. J Natl Cancer Inst 2004; 96: 1702–1713.
De Milito A, Iessi E, Logozzi MA, Lozupone F, Spada M, Marino ML et al. Proton pump inhibitors induce apoptosis of human B cell tumors through a caspase-independent mechanism involving reactive oxygen species. Cancer Res 2007; 67: 5408–5417.
de Gassart A, Gιminard C, Hoekstra D, Vidal M . Exosome secretion: the art of reutilizing nonrecycled proteins? Traffic 2004; 5: 896–903.
Zhang HG, Kim H, Liu C, Yu S, Wang J, Grizzle WE et al. Curcumin reverses breast tumor exosomes mediated immune suppression of NK cell tumor cytotoxicity. Biochim Biophys Acta 2007; 1773: 1116–11123.
Soiffer R, Lynch T, Mihm M, Jung K, Rhuda C, Schmollinger JC et al. Vaccination with irradiated autologous melanoma cells engineered to secrete human granulocyte–macrophage colony-stimulating factor generates potent antitumor immunity in patients with metastatic melanoma. Proc Natl Acad Sci USA 1998; 95: 13141–13146.
Palucka AK, Ueno H, Connolly J, Kerneis-Norvell F, Blanck JP, Johnston DA et al. Dendritic cells loaded with killed allogeneic melanoma cells can induce objective clinical responses and MART-1 specific CD8+ T-cell immunity. J Immunother (1997) 2006; 29: 545–557.
Maio M, Fonsatti E, Lamaj E, Altomonte M, Cattarossi I, Santantonio C et al. Vaccination of stage IV patients with allogeneic IL-4- or IL-2-gene-transduced melanoma cells generates functional antibodies against vaccinating and autologous melanoma cells. Cancer Immunol Immunother 2002; 51: 9–14.
Heiser A, Coleman D, Dannull J, Yancey D, Maurice MA, Lallas CD et al. Autologous dendritic cells transfected with prostate-specific antigen RNA stimulate CTL responses against metastatic prostate tumors. J Clin Invest 2002; 109: 409–417.
Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med 2007; 13: 54–61.
Muranski P, Boni A, Wrzesinski C, Citrin DE, Rosenberg SA, Childs R et al. Increased intensity lymphodepletion and adoptive immunotherapy – how far can we go? Nat Clin Pract Oncol 2006; 3: 668–681.
Peggs KS, Quezada SA, Korman AJ, Allison JP . Principles and use of anti-CTLA4 antibody in human cancer immunotherapy. Curr Opin Immunol 2006; 18: 206–213.
Frankel AE, Fleming DR, Powell BL, Gartenhaus R . DAB389IL2 (ONTAK) fusion protein therapy of chronic lymphocytic leukaemia. Exp Opin Biol Ther 2003; 3: 179–186.
Acknowledgements
We are grateful to Drs. Fabio Castiglioni and Erica Dugnani (Unit of Molecular Biology, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy) for the excellent graphical support, and to Dr. Antonello Villa (MIA Consortium, University of Bicocca, Milan, Italy) for scanning electron microscopy images. This work was supported by grants from the European Community Cancer Immunology and Immunotherapy contract no. 518234), the Italian Ministry of Industry, University and Research (Rome, Italy: grant FIRB RBNE017B4C), the Italian Association for Cancer Research. R Valenti was supported by a scholarship from the Foundation for Cancer Research (Milan, Italy).
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by G Kroemer
Rights and permissions
About this article
Cite this article
Iero, M., Valenti, R., Huber, V. et al. Tumour-released exosomes and their implications in cancer immunity. Cell Death Differ 15, 80–88 (2008). https://doi.org/10.1038/sj.cdd.4402237
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/sj.cdd.4402237
Keywords
This article is cited by
-
Advanced micro-/nanotechnologies for exosome encapsulation and targeting in regenerative medicine
Clinical and Experimental Medicine (2023)
-
Suppression of exosomal hsa_circ_0001005 eliminates the Vemurafenib resistance of melanoma
Journal of Cancer Research and Clinical Oncology (2023)
-
Microparticles: biogenesis, characteristics and intervention therapy for cancers in preclinical and clinical research
Journal of Nanobiotechnology (2022)
-
Cell membrane-camouflaged inorganic nanoparticles for cancer therapy
Journal of Nanobiotechnology (2022)
-
Molecular functions and therapeutic applications of exosomal noncoding RNAs in cancer
Experimental & Molecular Medicine (2022)