Abstract
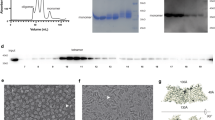
Some marine worms, such as Thelepus crispus and Notomastus lobatus, secrete brominated aromatic molecules and other halogenated metabolites as repellants1. Other species, such as Amphitrite ornata, do not produce repellants but are adapted to the chemical warfare of N. lobatus and cohabit with them in estuarine mudflats. The halocompounds are tolerated by A. ornata as they are degraded by dehaloperoxidase (DHP)2. We have determined the amino-acid sequence and crystal structure of DHP and find that its fold is typical of the globin family, indicating that the enzyme evolved from an oxygen carrier protein. Residues at the dimer interface do not correspond to those in tetrameric and dimeric haemoglobins and the spatial arrangement of the dimers is different. The complete amino-acid sequence is most similar to that of myoglobin from the sea hare, with 20.6% identity among 126 overlapping amino acids.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Woodin, S. A., Marinelli, R. L. & Lincoln, D. E. J. Chem. Ecol. 19, 517–530 (1993).
Chen, Y. P., Woodin, S. A., Lincoln, D. E. & Lovell, C. R. J. Biol. Chem. 271, 4609–4612 (1996).
Dawson, J. H. Science 240, 433–439 (1988).
Poulos, T. L. Adv. Inorg. Biochem. 7, 1–36 (1988).
Ator, M. A. & Ortiz de Montellano, P. R. J. Biol. Chem. 262, 1542–1551 (1987).
Matsui, T., Ozaki, S., Liong, E., Phillips, G. N. & Watanabe, Y. J. Biol. Chem. 274, 2838–2844 (1999).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lebioda, L., LaCount, M., Zhang, E. et al. An enzymatic globin from a marine worm. Nature 401, 445 (1999). https://doi.org/10.1038/46728
Issue date:
DOI: https://doi.org/10.1038/46728
This article is cited by
-
Selective tuning of activity in a multifunctional enzyme as revealed in the F21W mutant of dehaloperoxidase B from Amphitrite ornata
JBIC Journal of Biological Inorganic Chemistry (2018)
-
Degradation of sulfide by dehaloperoxidase-hemoglobin from Amphitrite ornata
JBIC Journal of Biological Inorganic Chemistry (2011)
-
The haemoglobin enzyme
Nature (1999)