Abstract
Aim:
Leflunomide is an immunosuppressive agent marketed as a disease-modifying antirheumatic drug. But it causes severe side effects, including fatal hepatitis and liver failure. In this study we investigated the contributions of hepatic metabolism and transport of leflunomide and its major metabolite teriflunomide to leflunomide induced hepatotoxicity in vitro and in vivo.
Methods:
The metabolism and toxicity of leflunomide and teriflunomide were evaluated in primary rat hepatocytes in vitro. Hepatic cytochrome P450 reductase null (HRN) mice were used to examine the PK profiling and hepatotoxicity of leflunomide in vivo. The expression and function of sodium/bile acid cotransporter (NTCP) were assessed in rat and human hepatocytes and NTCP-transfected HEK293 cells. After Male Sprague-Dawley (SD) rats were administered teriflunomide (1,6, 12 mg·kg−1·d−1, ig) for 4 weeks, their blood samples were analyzed.
Results:
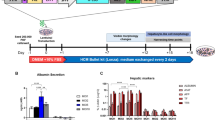
A nonspecific CYPs inhibitor aminobenzotriazole (ABT, 1 mmol/L) decreased the IC50 value of leflunomide in rat hepatocytes from 409 to 216 μmol/L, whereas another nonspecific CYPs inhibitor proadifen (SKF, 30 μmol/L) increased the cellular accumulation of leflunomide to 3.68-fold at 4 h. After oral dosing (15 mg/kg), the plasma exposure (AUC0-t) of leflunomide increased to 3-fold in HRN mice compared with wild type mice. Administration of leflunomide (25 mg·kg−1·d−1) for 7 d significantly increased serum ALT and AST levels in HRN mice; when the dose was increased to 50 mg·kg−1·d−1, all HRN mice died on d 6. Teriflunomide significantly decreased the expression of NTCP in human hepatocytes, as well as the function of NTCP in rat hepatocytes and NTCP-transfected HEK293 cells. Four-week administration of teriflunomide significantly increased serum total bilirubin and direct bilirubin levels in female rats, but not in male rats.
Conclusion:
Hepatic CYPs play a critical role in detoxification process of leflunomide, whereas the major metabolite teriflunomide suppresses the expression and function of NTCP, leading to potential cholestasis.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Alcorn N, Saunders S, Madhok R . Benefit-risk assessment of leflunomide: an appraisal of leflunomide in rheumatoid arthritis 10 years after licensing. Drug Saf 2009; 32: 1123–34.
Kalgutkar AS, Nguyen HT, Vaz AD, Doan A, Dalvie DK, McLeod DG, et al. In vitro metabolism studies on the isoxazole ring scission in the anti-inflammatory agent lefluonomide to its active alpha-cyanoenol metabolite A771726: mechanistic similarities with the cytochrome P450-catalyzed dehydration of aldoximes. Drug Metab Dispos 2003; 31: 1240–50.
Legras A, Bergemer-Fouquet AM, Jonville-Bera AP . Fatal hepatitis with leflunomide and itraconazole. Am J Med 2002; 113: 352–3.
Seah QM, New LS, Chan EC, Boelsterli UA . Oxidative bioactivation and toxicity of leflunomide in immortalized human hepatocytes and kinetics of the non-enzymatic conversion to its major metabolite, A77 1726. Drug Metab Lett 2008; 2: 153–7.
Shi Q, Yang X, Greenhaw J, Salminen WF . Hepatic cytochrome P450s attenuate the cytotoxicity induced by leflunomide and its active metabolite A77 1726 in primary cultured rat hepatocytes. Toxicol Sci 2011; 122: 579–86.
Confavreux C, Li DK, Freedman MS, Truffinet P, Benzerdjeb H, Wang D, et al. Long-term follow-up of a phase 2 study of oral teriflunomide in relapsing multiple sclerosis: safety and efficacy results up to 8.5 years. Mult Scler 2012; 18: 1278–89.
Miller AE, Wolinsky JS, Kappos L, Comi G, Freedman MS, Olsson TP, et al. Oral teriflunomide for patients with a first clinical episode suggestive of multiple sclerosis (TOPIC): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Neurol 2014; 13: 977–86.
Tallantyre E, Evangelou N, Constantinescu CS . Spotlight on teriflunomide. Int MS J 2008; 15: 62–8.
Kis E, Nagy T, Jani M, Molnár E, Jánossy J, Ujhellyi O, et al. Leflunomide and its metabolite A771726 are high affinity substrates of BCRP: implications for drug resistance. Ann Rheum Dis 2009; 68: 1201–7.
Jani M, Szabó P, Kis E, Molnár E, Glavinas H, Krajcsi P . Kinetic characterization of sulfasalazine transport by human ATP-binding cassette G2. Biol Pharm Bull 2009; 32: 497–9.
Chen ZS, Robey RW, Belinsky MG, Shchaveleva I, Ren XQ, Sugimoto Y, et al. Transport of methotrexate, methotrexate polyglutamates, and 17beta-estradiol 17-(beta-D-glucuronide) by ABCG2: effects of acquired mutations at R482 on methotrexate transport. Cancer Res 2003; 63: 4048–54.
Tiwari AK, Sodani K, Wang SR, Kuang YH, Ashby CR, Chen X, et al. Nilotinib (AMN107, Tasigna) reverses multidrug resistance by inhibiting the activity of the ABCB1/Pgp and ABCG2/BCRP/MXR transporters. Biochem Pharmacol 2009; 78: 153–61.
Loue C, Garnier N, Bertrand Y, Bleyzac N . High methotrexate exposure and toxicity in children with t(9;22) positive acute lymphoblastic leukaemia treated with imatinib. J Clin Pharm Ther 2015. doi:10.1111/jcpt.12298.
Gu J, Weng Y, Zhang QY, Cui H, Behr M, Wu L, et al. Liver-specific deletion of the NADPH-cytochrome P450 reductase gene: impact on plasma cholesterol homeostasis and the function and regulation of microsomal cytochrome P450 and heme oxygenase. J Biol Chem 2003; 278: 25895–901.
Liu X, LeCluyse EL, Brouwer KR, Lightfoot RM, Lee JI, Brouwer KL . Use of Ca2+ modulation to evaluate biliary excretion in sandwich-cultured rat hepatocytes. J Pharmacol Exp Ther 1999; 289: 1592–9.
Wu ZT, Qi XM, Sheng JJ, Ma LL, Ni X, Ren J, et al. Timosaponin A3 induces hepatotoxicity in rats through inducing oxidative stress and down-regulating bile acid transporters. Acta Pharmacol Sin 2014; 35: 1188–98.
Swift B, Pfeifer ND, Brouwer KL . Sandwich-cultured hepatocytes: an in vitro model to evaluate hepatobiliary transporter-based drug interactions and hepatotoxicity. Drug Metab Rev 2010; 42: 446–71.
Kanazawa H, Okada A, Igarashi E, Higaki M, Miyabe T, Sano T, et al. Determination of midazolam and its metabolite as a probe for cytochrome P450 3A4 phenotype by liquid chromatography-mass spectrometry. J Chromatogr A 2004; 1031: 213–8.
Chung JY, Kim JY, Kim WR, Lee SG, Kim YJ, Park JE, et al. Abundance of aryl hydrocarbon receptor potentiates benzo[a]pyrene-induced apoptosis in Hepa1c1c7 cells via CYP1A1 activation. Toxicology 2007; 235: 62–72.
McGinnity DF, Soars MG, Urbanowicz RA, Riley RJ . Evaluation of fresh and cryopreserved hepatocytes as in vitro drug metabolism tools for the prediction of metabolic clearance. Drug Metab Dispos 2004; 32: 1247–53.
Hu W, Sorrentino C, Denison MS, Kolaja K, Fielden MR . Induction of cyp1a1 is a nonspecific biomarker of aryl hydrocarbon receptor activation: results of large scale screening of pharmaceuticals and toxicants in vivo and in vitro. Mol Pharmacol 2007; 71: 1475–86.
O'Donnell EF, Kopparapu PR, Koch DC, Jang HS, Phillips JL, Tanguay RL, et al. The aryl hydrocarbon receptor mediates leflunomide-induced growth inhibition of melanoma cells. PLoS One 2012; 7: e40926.
O'Donnell EF, Saili KS, Koch DC, Kopparapu PR, Farrer D, Bisson WH, et al. The anti-inflammatory drug leflunomide is an agonist of the aryl hydrocarbon receptor. PLoS One 2010; 5. pii: e13128.
Bohanec Grabar P, Grabnar I, Rozman B, Logar D, Tomsic M, Suput D, et al. Investigation of the influence of CYP1A2 and CYP2C19 genetic polymorphism on 2-cyano-3-hydroxy-N-[4-(trifluoromethyl)phenyl]-2-butenamide (A77 1726) pharmacokinetics in leflunomide-treated patients with rheumatoid arthritis. Drug Metab Dispos 2009; 37: 2061–8.
Bohanec Grabar P, Rozman B, Tomsic M, Suput D, Logar D, Dolzan V . Genetic polymorphism of CYP1A2 and the toxicity of leflunomide treatment in rheumatoid arthritis patients. Eur J Clin Pharmacol 2008; 64: 871–6.
Wiese MD, Schnabl M, O'Doherty C, Spargo LD, Sorich MJ, Cleland LG, et al. Polymorphisms in cytochrome P450 2C19 enzyme and cessation of leflunomide in patients with rheumatoid arthritis. Arthritis Res Ther 2012; 14: R163.
Rozman B . Clinical pharmacokinetics of leflunomide. Clin Pharmacokinet 2002; 41: 421–30.
Padda MS, Sanchez M, Akhtar AJ, Boyer JL . Drug-induced cholestasis. Hepatology 2011; 53: 1377–87.
Dawson S, Stahl S, Paul N, Barber J, Kenna JG . In vitro inhibition of the bile salt export pump correlates with risk of cholestatic drug-induced liver injury in humans. Drug Metab Dispos 2012; 40: 130–8.
Fattinger K, Funk C, Pantze M, Weber C, Reichen J, Stieger B, et al. The endothelin antagonist bosentan inhibits the canalicular bile salt export pump: a potential mechanism for hepatic adverse reactions. Clin Pharmacol Ther 2001; 69: 223–31.
Vrenken TE, Buist-Homan M, Kalsbeek AJ, Faber KN, Moshage H . The active metabolite of leflunomide, A77 1726, protects rat hepatocytes against bile acid-induced apoptosis. J Hepatol 2008; 49: 799–809.
Rodrigues AD, Lai Y, Cvijic ME, Elkin LL, Zvyaga T, Soars MG . Drug-induced perturbations of the bile acid pool, cholestasis, and hepatotoxicity: mechanistic considerations beyond the direct inhibition of the bile salt export pump. Drug Metab Dispos 2014; 42: 566–74.
Henríquez-Hernández LA, Flores-Morales A, Santana-Farré R, Axelson M, Nilsson P, Norstedt G, et al. Role of pituitary hormones on 17alpha-ethinylestradiol-induced cholestasis in rat. J Pharmacol Exp Ther 2007; 320: 695–705.
Wang T, Zhou ZX, Sun LX, Li X, Xu ZM, Chen M, et al. Resveratrol effectively attenuates α-naphthyl-isothiocyanate-induced acute cholestasis and liver injury through choleretic and anti-inflammatory mechanisms. Acta Pharmacol Sin 2014; 35: 1527–36.
Li J, Yao HW, Jin Y, Zhang YF, Li CY, Li YH, et al. Pharmacokinetics of leflunomide in Chinese healthy volunteers. Acta Pharmacol Sin 2002; 23: 551–5.
Vinken M, Maes M, Vanhaecke T, Rogiers V . Drug-induced liver injury: mechanisms, types and biomarkers. Curr Med Chem 2013; 20: 3011–21.
Watanabe T, Miyake M, Shimizu T, Kamezawa M, Masutomi N, Shimura T, et al. Utility of bilirubins and bile acids as endogenous biomarkers for the inhibition of hepatic transporters. Drug Metab Dispos 2015; 43: 459–66.
Acknowledgements
We are grateful to Cinkate Pharmaceutical Intermediates Co, Ltd (Shanghai, China) for providing leflunomide and teriflunomide as gifts. We thank Dr Lei GUO (NCTR, USA) for helpful discussions and comments. We thank Dr Jun GU (New York State Department of Health, USA) for kindly providing the HRN mice.
This project is supported by the Key Projects of National Science and Technology Program, China (Grant No 2012ZX09301001-006, 012ZX09302003 and 2012ZX09301001), and National Science and Technology Major Project “Key New Drug Creation and Manufacturing Program”, China (Grant No 2012ZX09303-001).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Supplemental information is available at the Acta Pharmacologica Sinica's website.
Supplementary information
Supplemental Figure S1
Cytotoxicity of LEF and TER in primary rat hepatocytes. (JPG 149 kb)
Supplemental Figure S2
Effect of LEF and TER on the biliary excretion of d8-TCA. (JPG 224 kb)
Supplemental Methods
Measurement of the mitochondrial membrane potential (MMP) (DOC 126 kb)
Rights and permissions
About this article
Cite this article
Ma, Ll., Wu, Zt., Wang, L. et al. Inhibition of hepatic cytochrome P450 enzymes and sodium/bile acid cotransporter exacerbates leflunomide-induced hepatotoxicity. Acta Pharmacol Sin 37, 415–424 (2016). https://doi.org/10.1038/aps.2015.157
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/aps.2015.157
Keywords
This article is cited by
-
New equation to decipher the relationship between carbon isotopic composition of methane and maturity of gas source rocks
Science China Earth Sciences (2021)
-
Leflunomide increased the renal exposure of acyclovir by inhibiting OAT1/3 and MRP2
Acta Pharmacologica Sinica (2020)
-
Drug interactions in the treatment of rheumatoid arthritis and psoriatic arthritis
Rheumatology International (2020)
-
Formation mechanism of condensates, waxy and heavy oils in the southern margin of Junggar Basin, NW China
Science China Earth Sciences (2017)