Abstract
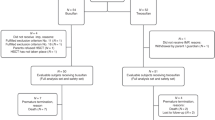
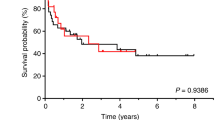
We report the long-term follow-up of children transplanted with Treosulfan (TREO)-based conditioning in Germany and Austria. Nine centres reported a total of 109 transplantations. Patients were stratified according to the paediatric TRM risk score derived from the paediatric BMT registry (PRST) and compared with the historical transplant population of this registry. Underlying diseases were malignancies, immunodeficiencies, and haematologic and metabolic disorders. TREO total dose ranged from 21–42 g/m2. Additional conditioning drugs included fludarabine, thiotepa, melphalan, CY and/or TBI. EFS at 3 years for non-malignant and malignant diseases was 88% and 49%, respectively. Leukaemia patients in remission had a survival of 51% at 3 years; nonremission patients relapsed and died within 18 months. TRM and OS in the low-risk groups 0 and 1 were similar to PRST controls. TRM in the high-risk groups 2 and 3 was markedly lower (9% vs 28% and 13% vs 53%, respectively) than in the PRST group, but OS was similar. In conclusion, TREO-based conditioning regimens in children resulted in excellent engraftment and long-term survival in nonmalignant disease. In high-risk malignancy, low acute toxicity was followed by low TRM but it did not translate into increased survival.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Casper J, Knauf W, Kiefer T, Wolff D, Steiner B, Hammer U et al. Treosulfan and fludarabine: a new toxicity-reduced conditioning regimen for allogeneic hematopoietic stem cell transplantation. Blood 2004; 103: 725–731.
Glowka FK, Romanski M, Wachowiak J . High-dose treosulfan in conditioning prior to hematopoietic stem cell transplantation. Expert. Opin Investig Drugs 2010; 19: 1275–1295.
Sauer M, Zeidler C, Meissner B, Rehe K, Hanke A, Welte K et al. Substitution of cyclophosphamide and busulfan by fludarabine, treosulfan and melphalan in a preparative regimen for children and adolescents with Shwachman-Diamond syndrome. Bone Marrow Transplant 2007; 39: 143–147.
Greystoke B, Bonanomi S, Carr TF, Gharib M, Khalid T, Coussons M et al. Treosulfan-containing regimens achieve high rates of engraftment associated with low transplant morbidity and mortality in children with non-malignant disease and significant co-morbidities. Br J Haematol 2008; 142: 257–262.
Cutting R, Mirelman A, Vora A . Treosulphan as an alternative to busulphan for myeloablative conditioning in paediatric allogeneic transplantation. Br J Haematol 2008; 143: 748–751.
Slatter MA, Rao K, Amrolia P, Flood T, Abinun M, Hambleton S et al. Treosulfan-based conditioning regimens for hematopoietic stem cell transplantation in children with primary immunodeficiency: United Kingdom experience. Blood 2011; 117: 4367–4375.
Wachowiak J, Sykora KW, Cornish J, Chybicka A, Kowalczyk JR, Gorczynska E et al. Treosulfan-based preparative regimens for allo-HSCT in childhood hematological malignancies: a retrospective study on behalf of the EBMT pediatric diseases working party. Bone Marrow Transplant 2011; 46: 1510–1518.
Matthes-Martin S, Potschger U, Bergmann K, Frommlet F, Brannath W, Bauer P et al. Risk-adjusted outcome measurement in pediatric allogeneic stem cell transplantation. Biol Blood Marrow Transplant 2008; 14: 335–343.
Kotlarz D, Beier R, Murugan D, Diestelhorst J, Jensen O et al. Loss of interleukin-10 signaling and infantile inflammatory bowel disease: implications for diagnosis and therapy. Gastroenterology 2012; 143: 347–355.
Wachowiak J, Grund G, Sykora KW, Wynn RF, Kowalczyk JR, Drabko K et al. Treosulfan-based conditioning regimen for allogeneic transplantation in children congenital non-malignant disorders—retrospective study. Bone Marrow Transplant 2008; 41: S80–S343.
Beier R, Schulz AS, Hoenig M, Schlegel G, Holter W, Stachel D et al. Treosulfan-based conditioning in children: retrospective analysis of the German and Austrian experience. Bone Marrow Transplant 2010; 45: S1–S11.
Poetschger U, Sykora KW, Zecca M, Veys P, Lankaster A, Cant A et al. Meta-analysis on treosulfan for conditioning in children and adolescents before haematopoietic stem cell transplantation. Bone Marrow Transplant 2012; 47: S1–S87.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant 1995; 15: 825–828.
Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant 2005; 11: 945–956.
Corbacioglu S, Greil J, Peters C, Wulffraat N, Laws HJ, Dilloo D et al. Defibrotide in the treatment of children with veno-occlusive disease (VOD): a retrospective multicentre study demonstrates therapeutic efficacy upon early intervention. Bone Marrow Transplant 2004; 33: 189–195.
Bernardo ME, Zecca M, Piras E, Vacca A, Giorgiani G, Cugno C et al. Treosulfan-based conditioning regimen for allogeneic haematopoietic stem cell transplantation in patients with thalassaemia major. Br J Haematol 2008; 143: 548–551.
Nemecek ER, Guthrie KA, Sorror ML, Wood BL, Doney KC, Hilger RA et al. Conditioning with treosulfan and fludarabine followed by allogeneic hematopoietic cell transplantation for high-risk hematologic malignancies. Biol Blood Marrow Transplant 2011; 17: 341–350.
Holowiecki J, Giebel S, Wojnar J, Krawczyk-Kulis M, Markiewicz M, Holowiecka-Goral A et al. Treosulfan and fludarabine low-toxicity conditioning for allogeneic haematopoietic stem cell transplantation in chronic myeloid leukaemia. Br J Haematol 2008; 142: 284–292.
Hilgendorf I, Wolff D, Gromke T, Trenschel R, Elmaagacli AH, Pichlmeier U et al. Retrospective analysis of treosulfan-based conditioning in comparison with standard conditioning in patients with myelodysplastic syndrome. Bone Marrow Transplant 2011; 46: 502–509.
Koenigsmann M, Mohren M, Jentsch-Ullrich K, Franke A, Becker E, Heim M et al. High-dose treosulfan in patients with relapsed or refractory high-grade lymphoma receiving tandem autologous blood stem cell transplantation. Bone Marrow Transplant 2004; 34: 477–483.
Chemnitz JM, von Lilienfeld-Toal M, Holtick U, Theurich S, Shimabukuro-Vornhagen A, Krause A et al. Intermediate intensity conditioning regimen containing FLAMSA, treosulfan, cyclophosphamide, and ATG for allogeneic stem cell transplantation in elderly patients with relapsed or high-risk acute myeloid leukaemia. Ann Hematol 2012; 91: 47–55.
Acknowledgements
This study was supported by the Integrated Research and Treatment Centre Transplantation (BMBF grant number 01E00802 IFB-TX, Hannover). We would like to give our special thanks to all the patients and their parents who took part in this study. Moreover, we want to thank all the physicians and nurses who have been involved in the transplantation and the follow-up care of the patients. We thank K Mischke and J Wolf for their diligent data management and S Blöss for patient management.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The following authors received financial support from Medac AG, Germany: Rita Beier, Ingo Müller and Karl-Walter Sykora research support. Karoline Ehlert, Ingo Müller and Karl-W Sykora travel grants. Meinolf Suttorp an educational grant. The other authors have no conflicts of interests in relation to this work to declare.
Rights and permissions
About this article
Cite this article
Beier, R., Schulz, A., Hönig, M. et al. Long-term follow-up of children conditioned with Treosulfan: German and Austrian experience. Bone Marrow Transplant 48, 491–501 (2013). https://doi.org/10.1038/bmt.2012.188
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/bmt.2012.188
Keywords
This article is cited by
-
Treosulfan vs busulfan conditioning for allogeneic bmt in children with nonmalignant disease: a randomized phase 2 trial
Bone Marrow Transplantation (2024)
-
Biallelic Form of a Known CD3E Mutation in a Patient with Severe Combined Immunodeficiency
Journal of Clinical Immunology (2020)
-
Treosulfan-based conditioning regimens for allogeneic haematopoietic stem cell transplantation in children with non-malignant diseases
Bone Marrow Transplantation (2015)
-
Alternative donor transplant of benign primary hematologic disorders
Bone Marrow Transplantation (2015)
-
Treosulfan-based conditioning regimens for allogeneic HSCT in children with acute lymphoblastic leukaemia
Annals of Hematology (2015)