Abstract
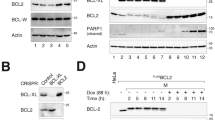
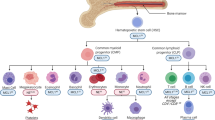
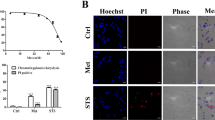
Anti-apoptotic Bcl-2 family proteins, which inhibit the mitochondrial pathway of apoptosis, are involved in the survival of various hematopoietic lineages and are often dysregulated in hematopoietic malignancies. However, their involvement in the megakaryocytic lineage is not well understood. In the present paper, we describe the crucial anti-apoptotic role of Mcl-1 and Bcl-xL in this lineage at multistages. The megakaryocytic lineage-specific deletion of both, in sharp contrast to only one of them, caused apoptotic loss of mature megakaryocytes in the fetal liver and systemic hemorrhage, leading to embryonic lethality. ABT-737, a Bcl-xL/Bcl-2/Bcl-w inhibitor, only caused thrombocytopenia in adult wild-type mice, but further induced massive mature megakaryocyte apoptosis in the Mcl-1 knockout mice, leading to severe hemorrhagic anemia. All these phenotypes were fully restored if Bak and Bax, downstream apoptosis executioners, were also deficient. In-vitro study revealed that the Jak pathway maintained Mcl-1 and Bcl-xL expression levels, preventing megakaryoblastic cell apoptosis. Similarly, both were involved in reticulated platelet survival, whereas platelet survival was dependent on Bcl-xL due to rapid proteasomal degradation of Mcl-1. In conclusion, Mcl-1 and Bcl-xL regulate the survival of the megakaryocytic lineage, which is critically important for preventing lethal or severe hemorrhage in both developing and adult mice.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- APS:
-
anti-platelet serum
- BH3:
-
Bcl-2 homology domain 3
- BM:
-
bone marrow
- ED:
-
embryonic day
- ER:
-
endoplasmic reticulum
- ET:
-
essential thrombocythemia
- Pf4:
-
platelet factor 4
- TPO:
-
thrombopoietin
- TUNEL:
-
terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick-end labeling
- VWF:
-
von Willebrand factor
References
Cory S, Adams JM . The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer 2002; 2: 647–656.
Opferman JT . Life and death during hematopoietic differentiation. Curr Opin Immunol 2007; 19: 497–502.
Opferman JT, Iwasaki H, Ong CC, Suh H, Mizuno S, Akashi K et al. Obligate role of anti-apoptotic MCL-1 in the survival of hematopoietic stem cells. Science 2005; 307: 1101–1104.
Dzhagalov I, St John A, He YW . The antiapoptotic protein Mcl-1 is essential for the survival of neutrophils but not macrophages. Blood 2007; 109: 1620–1626.
Steimer DA, Boyd K, Takeuchi O, Fisher JK, Zambetti GP, Opferman JT . Selective roles for antiapoptotic MCL-1 during granulocyte development and macrophage effector function. Blood 2009; 113: 2805–2815.
Motoyama N, Wang F, Roth KA, Sawa H, Nakayama K, Negishi I et al. Massive cell death of immature hematopoietic cells and neurons in Bcl-x-deficient mice. Science 1995; 267: 1506–1510.
Hamasaki A, Sendo F, Nakayama K, Ishida N, Negishi I, Hatakeyama S . Accelerated neutrophil apoptosis in mice lacking A1-a, a subtype of the bcl-2-related A1 gene. J Exp Med 1998; 188: 1985–1992.
Veis DJ, Sorenson CM, Shutter JR, Korsmeyer SJ . Bcl-2-deficient mice demonstrate fulminant lymphoid apoptosis, polycystic kidneys, and hypopigmented hair. Cell 1993; 75: 229–240.
Aichberger KJ, Mayerhofer M, Gleixner KV, Krauth MT, Gruze A, Pickl WF et al. Identification of MCL1 as a novel target in neoplastic mast cells in systemic mastocytosis: inhibition of mast cell survival by MCL1 antisense oligonucleotides and synergism with PKC412. Blood 2007; 109: 3031–3041.
Kozuma Y, Kojima H, Yuki S, Suzuki H, Nagasawa T . Continuous expression of Bcl-xL protein during megakaryopoiesis is post-translationally regulated by thrombopoietin-mediated Akt activation, which prevents the cleavage of Bcl-xL. J Thromb Haemost 2007; 5: 1274–1282.
Kodama T, Takehara T, Hikita H, Shimizu S, Shigekawa M, Li W et al. BH3-only activator proteins Bid and Bim are dispensable for Bak/Bax-dependent thrombocyte apoptosis induced by Bcl-xL deficiency: molecular requisites for the mitochondrial pathway to apoptosis in platelets. J Biol Chem 2011; 286: 13905–13913.
Mason KD, Carpinelli MR, Fletcher JI, Collinge JE, Hilton AA, Ellis S et al. Programmed anuclear cell death delimits platelet life span. Cell 2007; 128: 1173–1186.
Orlofsky A, Weiss LM, Kawachi N, Prystowsky MB . Deficiency in the anti-apoptotic protein A1-a results in a diminished acute inflammatory response. J Immunol 2002; 168: 1840–1846.
Print CG, Loveland KL, Gibson L, Meehan T, Stylianou A, Wreford N et al. Apoptosis regulator bcl-w is essential for spermatogenesis but appears otherwise redundant. Proc Natl Acad Sci USA 1998; 95: 12424–12431.
Kodama T, Takehara T, Hikita H, Shimizu S, Li W, Miyagi T et al. Thrombocytopenia exacerbates cholestasis-induced liver fibrosis in mice. Gastroenterology 2010; 138: 2487–2498; 2498 e1–7.
Josefsson EC, James C, Henley KJ, Debrincat MA, Rogers KL, Dowling MR et al. Megakaryocytes possess a functional intrinsic apoptosis pathway that must be restrained to survive and produce platelets. J Exp Med 2011; 208: 2017–2031.
Eisenberg-Lerner A, Bialik S, Simon HU, Kimchi A . Life and death partners: apoptosis, autophagy and the cross-talk between them. Cell Death Differ 2009; 16: 966–975.
Zinkel S, Gross A, Yang E . BCL2 family in DNA damage and cell cycle control. Cell Death Differ 2006; 13: 1351–1359.
Komatsu N, Suda T, Moroi M, Tokuyama N, Sakata Y, Okada M et al. Growth and differentiation of a human megakaryoblastic cell line, CMK. Blood 1989; 74: 42–48.
Kirito K, Watanabe T, Sawada K, Endo H, Ozawa K, Komatsu N . Thrombopoietin regulates Bcl-xL gene expression through Stat5 and phosphatidylinositol 3-kinase activation pathways. J Biol Chem 2002; 277: 8329–8337.
Kaushansky K . The molecular mechanisms that control thrombopoiesis. J Clin Invest 2005; 115: 3339–3347.
Deutsch VR, Tomer A . Megakaryocyte development and platelet production. Br J Haematol 2006; 134: 453–466.
Holmsen H . Physiological functions of platelets. Ann Med 1989; 21: 23–30.
Guthikonda S, Lev EI, Patel R, DeLao T, Bergeron AL, Dong JF et al. Reticulated platelets and uninhibited COX-1 and COX-2 decrease the antiplatelet effects of aspirin. J Thromb Haemost 2007; 5: 490–496.
Rinder HM, Tracey JB, Recht M, DeCastro L, Rinder CS, McHugh C et al. Differences in platelet alpha-granule release between normals and immune thrombocytopenic patients and between young and old platelets. Thromb Haemost 1998; 80: 457–462.
Harrison P, Robinson MS, Mackie IJ, Machin SJ . Reticulated platelets. Platelets 1997; 8: 379–383.
Tse C, Shoemaker AR, Adickes J, Anderson MG, Chen J, Jin S et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res 2008; 68: 3421–3428.
Vogler M, Hamali HA, Sun XM, Bampton ET, Dinsdale D, Snowden RT et al. BCL2/BCL-XL inhibition induces apoptosis, disrupts cellular calcium homeostasis, and prevents platelet activation. Blood 2011; 117: 7145–7154.
Rinkenberger JL, Horning S, Klocke B, Roth K, Korsmeyer SJ . Mcl-1 deficiency results in peri-implantation embryonic lethality. Genes Dev 2000; 14: 23–27.
Tiedt R, Schomber T, Hao-Shen H, Skoda RC . Pf4-Cre transgenic mice allow the generation of lineage-restricted gene knockouts for studying megakaryocyte and platelet function in vivo. Blood 2007; 109: 1503–1506.
Sanz C, Benet I, Richard C, Badia B, Andreu EJ, Prosper F et al. Antiapoptotic protein Bcl-x(L) is up-regulated during megakaryocytic differentiation of CD34(+) progenitors but is absent from senescent megakaryocytes. Exp Hematol 2001; 29: 728–735.
Hikita H, Takehara T, Shimizu S, Kodama T, Li W, Miyagi T et al. Mcl-1 and Bcl-xL cooperatively maintain integrity of hepatocytes in developing and adult murine liver. Hepatology 2009; 50: 1217–1226.
Debrincat MA, Josefsson EC, James C, Henley KJ, Ellis S, Lebois M et al. Mcl-1 and Bcl-xL co-ordinately regulate megakaryocyte survival. Blood 2012; 119: 5850–5858.
Mandelin AM, Pope RM . Myeloid cell leukemia-1 as a therapeutic target. Expert Opin Ther Targets 2007; 11: 363–373.
Masuda A, Matsuguchi T, Yamaki K, Hayakawa T, Yoshikai Y . Interleukin-15 prevents mouse mast cell apoptosis through STAT6-mediated Bcl-xL expression. J Biol Chem 2001; 276: 26107–26113.
Socolovsky M, Fallon AE, Wang S, Brugnara C, Lodish HF . Fetal anemia and apoptosis of red cell progenitors in Stat5a-/-5b-/- mice: a direct role for Stat5 in Bcl-X(L) induction. Cell 1999; 98: 181–191.
Kralovics R, Passamonti F, Buser AS, Teo SS, Tiedt R, Passweg JR et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med 2005; 352: 1779–1790.
Xing S, Wanting TH, Zhao W, Ma J, Wang S, Xu X et al. Transgenic expression of JAK2V617F causes myeloproliferative disorders in mice. Blood 2008; 111: 5109–5117.
Marty C, Lacout C, Martin A, Hasan S, Jacquot S, Birling MC et al. Myeloproliferative neoplasm induced by constitutive expression of JAK2V617F in knock-in mice. Blood 2010; 116: 783–787.
Panova-Noeva M, Marchetti M, Buoro S, Russo L, Leuzzi A, Finazzi G et al. JAK2V617F mutation and hydroxyurea treatment as determinants of immature platelet parameters in essential thrombocythemia and polycythemia vera patients. Blood 2011; 118: 2599–2601.
Kodama T, Takehara T, Hikita H, Shimizu S, Shigekawa M, Tsunematsu H et al. Increases in p53 expression induce CTGF synthesis by mouse and human hepatocytes and result in liver fibrosis in mice. J Clin Invest 2011; 121: 3343–3356.
Acknowledgements
We thank Radek Skoda (University Hospital Basel), Lothar Hennighausen (National Institutes of Health) and You-Wen He (Duke University) for providing the Pf4-Cre mice, the floxed bcl-x mice and the floxed mcl-1 mice, respectively. We thank Abbott Laboratories for providing ABT-737. This work was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology, Japan (to T Tak).
Author contributions
TK and TT designed the study and wrote the paper. HH, TK, MS, YH, WL and TM performed the mouse analyses. KK, ST and YT performed the in-vitro experiments and provided experimental advice. SS and TT performed the in-vitro experiments. AH, TK, NH and NH interpreted the data and provided experimental advice.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by P Bouillet
Supplementary information
Rights and permissions
About this article
Cite this article
Kodama, T., Hikita, H., Kawaguchi, T. et al. Mcl-1 and Bcl-xL regulate Bak/Bax-dependent apoptosis of the megakaryocytic lineage at multistages. Cell Death Differ 19, 1856–1869 (2012). https://doi.org/10.1038/cdd.2012.88
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cdd.2012.88
Keywords
This article is cited by
-
What can we learn from mice lacking pro-survival BCL-2 proteins to advance BH3 mimetic drugs for cancer therapy?
Cell Death & Differentiation (2022)
-
Hippo pathway-related genes expression is deregulated in myeloproliferative neoplasms
Medical Oncology (2022)
-
DMAG, a novel countermeasure for the treatment of thrombocytopenia
Molecular Medicine (2021)
-
The necroptotic cell death pathway operates in megakaryocytes, but not in platelet synthesis
Cell Death & Disease (2021)
-
The multiple mechanisms of MCL1 in the regulation of cell fate
Communications Biology (2021)