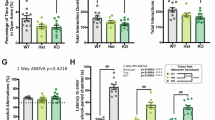
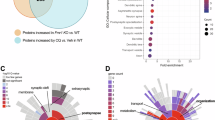
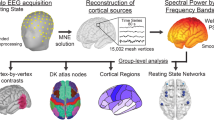
Abstract
Na+,K+-ATPase (NKA) is required to generate the resting membrane potential in neurons. Nociceptive afferent neurons express not only the α and β subunits of NKA but also the γ subunit FXYD2. However, the neural function of FXYD2 is unknown. The present study shows that FXYD2 in nociceptive neurons is necessary for maintaining the mechanical allodynia induced by peripheral inflammation. FXYD2 interacted with α1NKA and negatively regulated the NKA activity, depolarizing the membrane potential of nociceptive neurons. Mechanical allodynia initiated in FXYD2-deficient mice was abolished 4 days after inflammation, whereas it persisted for at least 3 weeks in wild-type mice. Importantly, the FXYD2/α1NKA interaction gradually increased after inflammation and peaked on day 4 post inflammation, resulting in reduction of NKA activity, depolarization of neuron membrane and facilitation of excitatory afferent neurotransmission. Thus, the increased FXYD2 activity may be a fundamental mechanism underlying the persistent hypersensitivity to pain induced by inflammation.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Geering K . The functional role of the β-subunit in the maturation and intracellular transport of Na,K-ATPase. FEBS Lett 1991; 285:189–193.
Noguchi S, Mishina M, Kawamura M, Numa S . Expression of functional (Na+ + K+)-ATPase from cloned cDNAs. FEBS Lett 1987; 225:27–32.
Jewell EA, Lingrel JB . Comparison of the substrate dependence properties of the rat Na,K-ATPase 1, 2, and 3 isoforms expressed in HeLa cells. J Biol Chem 1991; 266:16925–16930.
Therien AG, Blostein R . Mechanisms of sodium pump regulation. Am J Physiol Cell Physiol 2000; 279:C541–566.
Charnock JS, Post RL . Evidence of the mechanism of ouabain inhibition of cation-activated adenosine triphosphate. Nature 1963; 199:910–911.
Nesher M, Shpolansky U, Rosen H, Lichtstein D . The digitalis-like steroid hormones: new mechanisms of action and biological significance. Life Sci 2007; 80:2093–2107.
Li KC, Zhang FX, Li CL, et al. Follistatin-like 1 suppresses sensory afferent transmission by activating Na+,K+-ATPase. Neuron 2011; 69:974–987.
Li KC, Wang F, Zhong YQ, et al. Reduction of follistatin-like 1 in primary afferent neurons contributes to neuropathic pain hypersensitivity. Cell Res 2011; 21:697–699.
Crambert G, Geering K . FXYD proteins: new tissue-specific regulators of the ubiquitous Na,K-ATPase. Sci STKE 2003; 2003:RE1.
Sweadner KJ, Rael E . The FXYD gene family of small ion transport regulators or channels: cDNA sequence, protein signature sequence, and expression. Genomics 2000; 68:41–56.
Geering K . FXYD proteins: new regulators of Na-K-ATPase. Am J Physiol Renal Physiol 2006; 290:F241–F250.
Forbush B 3rd, Kaplan JH, Hoffman JF . Characterization of a new photoaffinity derivative of ouabain: labeling of the large polypeptide and of a proteolipid component of the Na, K-ATPase. Biochemistry 1978; 17:3667–3676.
Arystarkhova E, Wetzel RK, Asinovski NK, Sweadner KJ . The γ subunit modulates Na+ and K+ affinity of the renal Na,K-ATPase. J Biol Chem 1999; 274:33183–33185.
Beguin P, Crambert G, Guennoun S, Garty H, Horisberger JD, Geering K . CHIF, a member of the FXYD protein family, is a regulator of Na,K-ATPase distinct from the γ-subunit. EMBO J 2001; 20:3993–4002.
Beguin P, Wang X, Firsov D, et al. The γ subunit is a specific component of the Na,K-ATPase and modulates its transport function. EMBO J 1997; 16:4250–4260.
Maxwell DJ, Réthelyi M . Ultrastructure and synaptic connections of cutaneous afferent fibres in the spinal cord. Trends Neurosci 1987; 10:117–123.
Woolf CJ, Ma Q . Nociceptors-noxious stimulus detectors. Neuron 2007; 55:353–364.
Gold MS, Gebhart GF . Nociceptor sensitization in pain pathogenesis. Nat Med 2010; 16:1248–1257.
Fink DJ, Fang D, Li T, Mata M . Na,K-ATPase β subunit isoform expression in the peripheral nervous system of the rat. Neurosci Lett 1995; 183:206–209.
Mata M, Siegel GJ, Hieber V, Beaty MW, Fink DJ . Differential distribution of (Na,K)-ATPase α isoform mRNAs in the peripheral nervous system. Brain Res 1991; 546:47–54.
Dobretsov M, Hastings SL, Stimers JR . Non-uniform expression of α subunit isoforms of the Na+/K+ pump in rat dorsal root ganglia neurons. Brain Res 1999; 821:212–217.
Dobretsov M, Hastings SL, Stimers JR . Functional Na+/K+ pump in rat dorsal root ganglia neurons. Neuroscience 1999; 93:723–729.
Venteo S, Bourane S, Mechaly I, et al. Regulation of the Na,K-ATPase gamma-subunit FXYD2 by Runx1 and Ret signaling in normal and injured non-peptidergic nociceptive sensory neurons. PLoS One 2012; 7:e29852.
Vrontou S, Wong AM, Rau KK, Koerber HR, Anderson DJ . Genetic identification of C fibres that detect massage-like stroking of hairy skin in vivo. Nature 2013; 493:669–673.
Li L, Rutlin M, Abraira VE, et al. The functional organization of cutaneous low-threshold mechanosensory neurons. Cell 2011; 147:1615–1627.
Ataka T, Kumamoto E, Shimoji K, Yoshimura M . Baclofen inhibits more effectively C-afferent than Aδ-afferent glutamatergic transmission in substantia gelatinosa neurons of adult rat spinal cord slices. Pain 2000; 86:273–282.
Bird GC, Han JS, Fu Y, Adwanikar H, Willis WD, Neugebauer V . Pain-related synaptic plasticity in spinal dorsal horn neurons: role of CGRP. Mol Pain 2006; 2:31.
Masocha W, Horvath G, Agil A, et al. Role of Na+, K+-ATPase in morphine-induced antinociception. J Pharmacol Exp Ther 2003; 306:1122–1128.
Woolcock K, Specht SC . Modulation of Na, K-ATPase activity by prostaglandin E1 and [D-Ala2,N-Me-Phe4,Gly5-ol]-enkephalin. Life Sci 2006; 78:1653–1661.
Feraille E, Doucet A . Sodium-potassium-adenosinetriphosphatase-dependent sodium transport in the kidney: hormonal control. Physiol Rev 2001; 81:345–418.
Aizman O, Aperia A . Na+,K+-ATPase as a signal transducer. Ann NY Acad Sci 2003; 986:489–496.
Albers RWS, Siegel GJ . The ATP-Dependent Na+, K+ Pump. in: Basic neurochemistry: molecular, cellular, and medical aspects. 6th Edition. Philadelphia: Lippincott-Raven 1999.
Scuri R, Lombardo P, Cataldo E, Ristori C, Brunelli M . Inhibition of Na+/K+ ATPase potentiates synaptic transmission in tactile sensory neurons of the leech. Eur J Neurosci 2007; 25:159–167.
Arystarkhova E, Wetzel RK . Gamma structural variants differentially regulate Na,K-ATPase properties. Ann NY Acad Sci 2003; 986:416–419.
Arystarkhova E, Sweadner KJ . Splice variants of the gamma subunit (FXYD2) and their significance in regulation of the Na, K-ATPase in kidney. J Bioenerg Biomembr 2005; 37:381–386.
Bibert S, Liu CC, Figtree GA, et al. FXYD proteins reverse inhibition of the Na+-K+ pump mediated by glutathionylation of its β1 subunit. J Biol Chem 2011; 286:18562–18572.
Ferrari LF, Bogen O, Levine JD . Nociceptor subpopulations involved in hyperalgesic priming. Neuroscience 2010; 165:896–901.
Ferrari LF, Bogen O, Levine JD . Role of nociceptor αCaMKII in transition from acute to chronic pain (hyperalgesic priming) in male and female rats. J Neurosci 2013; 33:11002–11011.
Joseph EK, Levine JD . Mu and delta opioid receptors on nociceptors attenuate mechanical hyperalgesia in rat. Neuroscience 2010; 171:344–350.
Liu XJ, Zhang FX, Liu H, et al. Activin C expressed in nociceptive afferent neurons is required for suppressing inflammatory pain. Brain 2012; 135:391–403.
Zhang FX, Liu XJ, Gong LQ, et al. Inhibition of inflammatory pain by activating B-type natriuretic peptide signal pathway in nociceptive sensory neurons. J Neurosci 2010; 30:10927–10938.
Jones DH, Li TY, Arystarkhova E, et al. Na,K-ATPase from mice lacking the γ subunit (FXYD2) exhibits altered Na+ affinity and decreased thermal stability. J Biol Chem 2005; 280:19003–19011.
Wu ZQ, Li M, Chen J, Chi ZQ, Liu JG . Involvement of cAMP/cAMP-dependent protein kinase signaling pathway in regulation of Na+,K+-ATPase upon activation of opioid receptors by morphine. Mol Pharmacol 2006; 69:866–876.
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ . Protein measurement with the folin phenol reagent. J Biol Chem 1951; 193:265–275.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (31130066) and the Strategic Priority Research Program (B) of Chinese Academy of Sciences (XDB01020300).
Author information
Authors and Affiliations
Corresponding author
Additional information
( Supplementary information is linked to the online version of the paper on the Cell Research website.)
Supplementary information
Supplementary information, Figure S1
Recording of membrane potential in IB4-negative small DRG neurons. (PDF 39 kb)
Supplementary information, Figure S2
Recording of AP and AHP in IB4-positive small DRG neurons. (PDF 39 kb)
Supplementary information, Figure S3
Expression of α1 and β1 subunits of NKA, FSTL1 and FXYD2 in DRGs after peripheral inflammation. (PDF 135 kb)
Supplementary information, Figure S4
Time course of NKA activity in ATP-treated microsomes. (PDF 36 kb)
Supplementary information, Data S1
Materials and Methods (PDF 42 kb)
Rights and permissions
About this article
Cite this article
Wang, F., Cai, B., Li, KC. et al. FXYD2, a γ subunit of Na+,K+-ATPase, maintains persistent mechanical allodynia induced by inflammation. Cell Res 25, 318–334 (2015). https://doi.org/10.1038/cr.2015.12
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cr.2015.12
Keywords
This article is cited by
-
Single cell multiomic analysis reveals diabetes-associated β-cell heterogeneity driven by HNF1A
Nature Communications (2023)
-
FXYD2 mRNA expression represents a new independent factor that affects survival of glioma patients and predicts chemosensitivity of patients to temozolomide
BMC Neurology (2021)
-
Effect of a purine derivative containing selenium to improve memory decline and anxiety through modulation of the cholinergic system and Na+/K+-ATPase in an Alzheimer’s disease model
Metabolic Brain Disease (2021)
-
The spinal microglial IL-10/β-endorphin pathway accounts for cinobufagin-induced mechanical antiallodynia in bone cancer pain following activation of α7-nicotinic acetylcholine receptors
Journal of Neuroinflammation (2020)
-
High-dose maternal folic acid supplementation before conception impairs reversal learning in offspring mice
Scientific Reports (2017)