Abstract
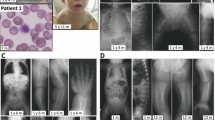
A rare lethal autosomal recessive syndrome with skeletal dysplasia, polycystic kidneys and multiple malformations was first described by Gillessen-Kaesbach et al and subsequently by Nishimura et al. The skeletal features uniformly comprise a round pelvis, mesomelic shortening of the upper limbs and defective ossification of the cervical spine. We studied two unrelated families including three affected fetuses with Gillessen-Kaesbach–Nishimura syndrome using whole-exome and Sanger sequencing, comparative genome hybridization and homozygosity mapping. All affected patients were shown to have a novel homozygous splice variant NM_024740.2: c.1173+2T>A in the ALG9 gene, encoding alpha-1,2-mannosyltransferase, involved in the formation of the lipid-linked oligosaccharide precursor of N-glycosylation. RNA analysis demonstrated skipping of exon 10, leading to shorter RNA. Mass spectrometric analysis showed an increase in monoglycosylated transferrin as compared with control tissues, confirming that this is a congenital disorder of glycosylation (CDG). Only three liveborn children with ALG9-CDG have been previously reported, all with missense variants. All three suffered from intellectual disability, muscular hypotonia, microcephaly and renal cysts, but none had skeletal dysplasia. Our study shows that some pathogenic variants in ALG9 can present as a lethal skeletal dysplasia with visceral malformations as the most severe phenotype. The skeletal features overlap with that previously reported for ALG3- and ALG12-CDG, suggesting that this subset of glycosylation disorders constitutes a new diagnostic group of skeletal dysplasias.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Gillessen-Kaesbach G, Meinecke P, Garrett C et al: New autosomal recessive lethal disorder with polycystic kidneys type Potter I, characteristic face, microcephaly, brachymelia, and congenital heart defects. Am J Med Genet 1993; 45: 511–518.
Nishimura G, Nakayama M, Fuke Y et al: A lethal osteochondrodysplasia with mesomelic brachymelia, round pelvis, and congenital hepatic fibrosis: two siblings born to consanguineous parents. Pediatr Radiol 1998; 28: 43–47.
Jaeken J : Congenital disorders of glycosylation (CDG): it's (nearly) all in it!. J Inherit Metab Dis 2011; 34: 853–858.
Freeze HH, Eklund EA, Ng BG et al: Neurology of inherited glycosylation disorders. Lancet Neurol 2012; 11: 453–466.
Coman D, Irving M, Kannu P et al: The skeletal manifestations of the congenital disorders of glycosylation. Clin Genet 2008; 73: 507–515.
Murali C, Lu JT, Jain M et al: Diagnosis of ALG12-CDG by exome sequencing in a case of severe skeletal dysplasia. Mol Genet Metab Rep 2014; 1: 213–219.
Kranz C, Basinger AA, Gucsavas-Calikoglu M et al: Expanding spectrum of congenital disorder of glycosylation Ig (CDG-Ig): sibs with a unique skeletal dysplasia, hypogammaglobulinemia, cardiomyopathy, genital malformations, and early lethality. Am J Med Genet A 2007; 143A: 1371–1378.
Frank CG, Grubenmann CE, Eyaid W et al: Identification and functional analysis of a defect in the human ALG9 gene: definition of congenital disorder of glycosylation type IL. Am J Hum Genet 2004; 75: 146–150.
Freeze HH, Chong JX, Bamshad MJ et al: Solving glycosylation disorders: fundamental approaches reveal complicated pathways. Am J Hum Genet 2014; 94: 161–175.
Weinstein M, Schollen E, Matthijs G et al: CDG-IL: an infant with a novel mutation in the ALG9 gene and additional phenotypic features. Am J Med Genet A 2005; 136: 194–197.
Vleugels W, Keldermans L, Jaeken J et al: Quality control of glycoproteins bearing truncated glycans in an ALG9-defective (CDG-IL) patient. Glycobiology 2009; 19: 910–917.
Sun L, Eklund EA, Chung WK et al: Congenital disorder of glycosylation id presenting with hyperinsulinemic hypoglycemia and islet cell hyperplasia. J Clin Endocrinol Metab 2005; 90: 4371–4375.
Li H, Durbin R : Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 2009; 25: 1754–1760.
DePristo MA, Banks E, Poplin R et al: A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat Genet 2011; 43: 491–498.
Lacey JM, Bergen HR, Magera MJ et al: Rapid determination of transferrin isoforms by immunoaffinity liquid chromatography and electrospray mass spectrometry. Clin Chem 2001; 47: 513–518.
Iqbal Z, Vandeweyer G, van der Voet M et al: Homozygous and heterozygous disruptions of ANK3: at the crossroads of neurodevelopmental and psychiatric disorders. Hum Mol Genet 2013; 22: 1960–1970.
Oriol R, Martinez-Duncker I, Chantret I et al: Common origin and evolution of glycosyltransferases using Dol-P-monosaccharides as donor substrate. Mol Biol Evol 2002; 19: 1451–1463.
van Rooijen JJ, Jeschke U, Kamerling JP et al: Expression of N-linked sialyl Le(x) determinants and O-glycans in the carbohydrate moiety of human amniotic fluid transferrin during pregnancy. Glycobiology 1998; 8: 1053–1064.
Stanley P, Schachter H, Taniguchi N : Essentials of Glycobiology. NY, USA: Cold Spring Harbor Laboratory Press, 2009.
Zielinska DF, Gnad F, Wisniewski JR et al: Precision mapping of an in vivo N-glycoproteome reveals rigid topological and sequence constraints. Cell 2010; 141: 897–907.
Niklasson A, Albertsson-Wikland K : Continuous growth reference from 24th week of gestation to 24 months by gender. BMC Pediatr 2008; 8: 8.
Voigt M, Fusch C, Olbertz D et al: Analyse des neugeborenenkollektivs der bundesrepublik deutschland. Geburtsh Frauenheilk 2006; 66: 956–970.
Acknowledgements
We thank our patients and their families for participating in this study, Professor Magnus Nordenskjöld for generous support and nice environment at the laboratory and Professor Matthew Warman for advice regarding this manuscript. We also thank laboratory engineers and technicians Isabel Neira, Weini Tadesse, Malin Hertzman, Anette Niklasson, Annica Westlund, Christine Carlsson-Skwirut, Britt Masironi and Nina Jäntti for assistance. GG, AN and ET have been supported through the regional agreement on medical training and clinical research (ALF) between Stockholm County Council (531315) and Karolinska Institutet, by grants from Kronprinsessan Lovisa and Axel Tiellmans Minnesfond, Stiftelsen Samariten, Frimurare Barnhuset i Stockholm, Karolinska Institutet, Promobilia Foundation and The Swedish Childhood Cancer Foundation. EAE received grants from the Crafoord Foundation and Kungl. Fysiografiska Sällskapet in Lund.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on European Journal of Human Genetics website
Supplementary information
Rights and permissions
About this article
Cite this article
Tham, E., Eklund, E., Hammarsjö, A. et al. A novel phenotype in N-glycosylation disorders: Gillessen-Kaesbach–Nishimura skeletal dysplasia due to pathogenic variants in ALG9. Eur J Hum Genet 24, 198–207 (2016). https://doi.org/10.1038/ejhg.2015.91
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ejhg.2015.91
This article is cited by
-
Cystic kidney diseases associated with mutations in phosphomannomutase 2 promotor: a large spectrum of phenotypes
Pediatric Nephrology (2021)
-
RETRACTED ARTICLE: Aberrant mannosylation profile and FTX/miR-342/ALG3-axis contribute to development of drug resistance in acute myeloid leukemia
Cell Death & Disease (2018)
-
Recognizable phenotypes in CDG
Journal of Inherited Metabolic Disease (2018)
-
ALG9 Associated Gillessen-Kaesbach–Nishimura Syndrome (GIKANIS): An Uncommon Aetiology of Enlarged Foetal Kidneys
Journal of Fetal Medicine (2018)
-
Abnormal glycosylation in Joubert syndrome type 10
Cilia (2017)