Abstract
Purpose
Epithelial mesenchymal transition (EMT) plays a central role in the development of fibrotic complications of the lens. The current study is designed to check whether EMT of lens epithelial cells (LECs) is regulated by epigenetic modifications and to evaluate the effect of Trichostatin-A (TSA) on the transforming growth factor-β (TGF-β)-induced EMT.
Methods
Fetal human LECs (FHL124) were treated with TGF-β2 in the presence or absence of TSA. Levels of mRNA, protein, as well as localization of α-smooth muscle actin (αSMA) were studied along with migration of LECs. Acetylation of histone H4 was analyzed and chromatin immunoprecipitation (ChIP) was carried out to study the level of acetylated histone H4 at the promoter of αSMA gene (ACTA2). Student’s t-test was used for statistical analysis.
Results
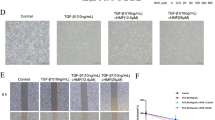
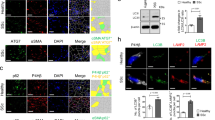
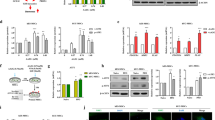
TGF-β2 treatment resulted in myofibroblast-like changes and increased migratory capacity of FHL124. Protein and mRNA expression of αSMA increased, and immunofluorescence revealed presence of extensive stress fibers. TSA treatment preserved epithelial morphology, retarded cell migration, and abrogated an increase in αSMA levels. TSA led to the accumulation of acetylated histone H4 that was reduced on TGF-β2 treatment. However, increased level of histone H4 acetylation was found at the ACTA2 promoter region during TGF-β treatment.
Conclusions
The increased level of αSMA, a hallmark of EMT in LECs, is associated with increased level of histone H4 acetylation at its promoter region, and TSA helps in suppressing EMT by epigenetically reducing this level. TSA thus shows promising potential in management of fibrotic conditions of the lens.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Kalluri R, Weinberg RA . The basics of epithelial-mesenchymal transition. J Clin Invest 2009; 119 (6): 1420–1428.
Srinivasan Y, Lovicu FJ, Overbeek PA . Lens-specific expression of transforming growth factor beta1 in transgenic mice causes anterior subcapsular cataracts. J Clin Invest 1998; 101 (3): 625–634.
Wormstone IM, Tamiya S, Anderson I, Duncan G . TGF-beta2-induced matrix modification and cell transdifferentiation in the human lens capsular bag. Invest Ophthalmol Vis Sci 2002; 43 (7): 2301–2308.
Lovicu FJ, Schulz MW, Hales AM, Vincent LN, Overbeek PA, Chamberlain CG et al. TGFbeta induces morphological and molecular changes similar to human anterior subcapsular cataract. Br J Ophthalmol 2002; 86 (2): 220–226.
Apple DJ, Solomon KD, Tetz MR, Assia EI, Holland EY, Legler UF et al. Posterior capsule opacification. Surv Ophthalmol 1992; 37 (2): 73–116.
Hodge WG . Posterior capsule opacification after cataract surgery. Ophthalmology 1998; 105 (6): 943–944.
Raj SM, Vasavada AR, Johar SR, Vasavada VA . Post-operative capsular opacification: a review. Int J Biomed Sci 2007; 3 (4): 237–250.
Buckley EG, Klombers LA, Seaber JH, Scalise-Gordy A, Minzter R . Management of the posterior capsule during pediatric intraocular lens implantation. Am J Ophthalmol 1993; 115 (6): 722–728.
Glenisson W, Castronovo V, Waltregny D . Histone deacetylase 4 is required for TGFbeta1-induced myofibroblastic differentiation. Biochim Biophys Acta 2007; 1773 (10): 1572–1582.
Guo W, Shan B, Klingsberg RC, Qin X, Lasky JA . Abrogation of TGF-beta1-induced fibroblast-myofibroblast differentiation by histone deacetylase inhibition. Am J Physiol Lung Cell Mol Physiol 2009; 297 (5): L864–L870.
Cho JS, Moon YM, Park IH, Um JY, Moon JH, Park SJ et al. Epigenetic regulation of myofibroblast differentiation and extracellular matrix production in nasal polyp-derived fibroblasts. Clin Exp Allergy 2012; 42 (6): 872–882.
Barter MJ, Pybus L, Litherland GJ, Rowan AD, Clark IM, Edwards DR et al. HDAC-mediated control of ERK- and PI3K-dependent TGF-beta-induced extracellular matrix-regulating genes. Matrix Biol 2010; 29 (7): 602–612.
Struhl K . Histone acetylation and transcriptional regulatory mechanisms. Genes Dev 1998; 12 (5): 599–606.
Lei W, Zhang K, Pan X, Hu Y, Wang D, Yuan X et al. Histone deacetylase 1 is required for transforming growth factor-beta1-induced epithelial-mesenchymal transition. Int J Biochem Cell Biol 2010; 42 (9): 1489–1497.
Yoshida M, Kijima M, Akita M, Beppu T . Potent and specific inhibition of mammalian histone deacetylase both in vivo and in vitro by trichostatin A. J Biol Chem 1990; 265 (28): 17174–17179.
Kaimori A, Potter JJ, Choti M, Ding Z, Mezey E, Koteish AA . Histone deacetylase inhibition suppresses the transforming growth factor beta1-induced epithelial-to-mesenchymal transition in hepatocytes. Hepatology 2010; 52 (3): 1033–1045.
Zhou Q, Wang Y, Yang L, Chen P, Dong X, Xie L . Histone deacetylase inhibitors blocked activation and caused senescence of corneal stromal cells. Mol Vis 2008; 14: 2556–2565.
Sharma A, Mehan MM, Sinha S, Cowden JW, Mohan RR . Trichostatin a inhibits corneal haze in vitro and in vivo. Invest Ophthalmol Vis Sci 2009; 50 (6): 2695–2701.
Kitano A, Okada Y, Yamanka O, Shirai K, Mohan RR, Saika S . Therapeutic potential of trichostatin A to control inflammatory and fibrogenic disorders of the ocular surface. Mol Vis 2010; 16: 2964–2973.
Chen X, Xiao W, Chen W, Luo L, Ye S, Liu Y . The epigenetic modifier trichostatin A, a histone deacetylase inhibitor, suppresses proliferation and epithelial-mesenchymal transition of lens epithelial cells. Cell Death Dis 2013; 4: e884.
Rungger-Brandle E, Conti A, Leuenberger PM, Rungger D . Expression of alphasmooth muscle actin in lens epithelia from human donors and cataract patients. Exp Eye Res 2005; 81 (5): 539–550.
Hales AM, Schulz MW, Chamberlain CG, McAvoy JW . TGF-beta 1 induces lens cells to accumulate alpha-smooth muscle actin, a marker for subcapsular cataracts. Curr Eye Res 1994; 13 (12): 885–890.
Ganatra DA, Johar KS, Parmar TJ, Patel AR, Rajkumar S, Arora AI et al. Estrogen mediated protection of cytoskeleton against oxidative stress. Indian J Med Res 2013; 137 (1): 117–124.
Jayani RS, Ramanujam PL, Galande S . Studying histone modifications and their genomic functions by employing chromatin immunoprecipitation and immunoblotting. Methods Cell Biol 2010; 98: 35–56.
Jampel HD, Roche N, Stark WJ, Roberts AB . Transforming growth factor-beta in human aqueous humor. Curr Eye Res 1990; 9 (10): 963–969.
Wormstone IM, Tamiya S, Eldred JA, Lazaridis K, Chantry A, Reddan JR et al. Characterisation of TGF-beta2 signalling and function in a human lens cell line. Exp Eye Res 2004; 78 (3): 705–714.
Gabbiani G . The myofibroblast in wound healing and fibrocontractive diseases. J Pathol 2003; 200 (4): 500–503.
Jester JV, Petroll WM, Barry PA, Cavanagh HD . Expression of alpha-smooth muscle (alpha-SM) actin during corneal stromal wound healing. Invest Ophthalmol Vis Sci 1995; 36 (5): 809–819.
Darby I, Skalli O, Gabbiani G . Alpha-smooth muscle actin is transiently expressed by myofibroblasts during experimental wound healing. Lab Invest 1990; 63 (1): 21–29.
Kakudo N, Kushida S, Suzuki K, Ogura T, Notodihardjo PV, Hara T et al. Effects of transforming growth factor-beta1 on cell motility, collagen gel contraction, myofibroblastic differentiation, and extracellular matrix expression of human adipose-derived stem cell. Hum Cell 2012; 25 (4): 87–95.
Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA . Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol 2002; 3 (5): 349–363.
Huang L . Targeting histone deacetylases for the treatment of cancer and inflammatory diseases. J Cell Physiol 2006; 209 (3): 611–616.
Acknowledgements
This work was supported by WOS-A scheme of the Department of Science and Technology (SR/WOS-A/LS-272/2010). We thank Dr K Thangaraj of Centre for Cellular and Molecular Biology, Hyderabad, India, for his help with STR profiling. This paper was presented in part at the Asia-ARVO 2013 conference, New Delhi, India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on Eye website
Rights and permissions
About this article
Cite this article
Ganatra, D., Rajkumar, S., Patel, A. et al. Association of histone acetylation at the ACTA2 promoter region with epithelial mesenchymal transition of lens epithelial cells. Eye 29, 828–838 (2015). https://doi.org/10.1038/eye.2015.29
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/eye.2015.29
This article is cited by
-
Regulation of epithelial-mesenchymal transition by protein lysine acetylation
Cell Communication and Signaling (2022)
-
AGE-RAGE interaction in the TGFβ2-mediated epithelial to mesenchymal transition of human lens epithelial cells
Glycoconjugate Journal (2016)