Abstract
This study investigated the anatomical integrity of the vagal innervation to the gastrointestinal tract following Roux-en-Y gastric bypass (RYGB) in the mouse. Specifically, the surgical procedure was performed in high-fat-fed reporter mice (Phox2b-Cre-tdTomato), in which the entire vagal innervation of the gastrointestinal tract was fluorescently labeled. As a result, our anatomical observations revealed both qualitative and quantitative changes of the vagal supply to the gut after RYGB. This included the extensive denervation of the glandular and distal stomach, and sites of surgical interventions (clipping and anastomosis). Furthermore, the stomach wall after RYGB frequently contained dystrophic axons and endings, suggestive of vagal neurodegeneration. In contrast, RYGB did not significantly modify the innervation to the rest of the intestines and glucostatic organs. In summary, the present study describes a previously unrecognized pattern of vagal remodeling and denervation following RYGB. Our findings may serve as a guideline for future investigations on the role of gut–brain communication in bariatric surgery.
Similar content being viewed by others
Introduction
The remarkable efficacy of Roux-en-Y gastric bypass (RYGB) in reversing morbid obesity and diabetes is well documented.1 Recent studies reported that subsets of vagal neurons are critically implicated in the antiobesity2 and antidiabetic actions of bariatric surgery in rodents.3, 4, 5 In addition, many of the unwanted side effects of RYGB, such as dumping syndrome, are suggestive of altered vagal communication.6 Finally, whereas surgeons are careful not to cut the vagus nerve during RYGB, it is a reasonable assumption that the extrinsic innervation of the stomach and intestines is interrupted at the different sites of surgical intervention.7
All the aforementioned observations combined together strongly suggest that RYGB may have a direct impact on the anatomical integrity of the vagal innervation to the gastrointestinal tract, which in turn may have a direct role in the beneficial and/or side effects of the surgery. However, there has never been a study on the integrity of the vagus nerve following weight-loss surgery because of the combined technical challenges of performing RYGB and labeling vagal neurons in laboratory animals. The current study sought to systematically survey the impact of RYGB on vagal innervation of the gastrointestinal tract and abdominal viscera in a fluorescent protein reporter mouse model, providing us an efficient manner of labeling all vagal neurons. Importantly, the surgical procedure used in this study is analogous to that performed in morbidly obese patients in terms of metabolic outcome and surgical technique.5, 8 Therefore, we strongly believe that our findings have direct implications for the bariatric surgeon.
Methods
Transgenic mice
Phox2b-Cre mice on a C57Bl/6 genetic background were generated as previously described9 and crossed with tdTomato reporter mice from the Jackson Laboratory (Bar Harbor, ME, USA; stock# 007905). This mouse line was chosen because it expresses Cre in all the vagal neurons (afferent and efferent), but not in sympathetic ganglia and spinal sensory neurons. The Cre allele was detected using the following primers: 5’-CCGTCTCCACATCCATCTTT-3′ (Phox2bF2), 5′-CTACGGACTCTGGTGGT-3′ (Phox2bR2), 5′-ATTCTCCCACCGTCACTACG-3′ (CreR2). All the mice used in our study were adult males carrying one Phox2b-Cre allele and one floxed-tdTomato allele (Phox2b-Cre-tdTomato) housed in a light-controlled (12 h on/12 h off; lights on at 0700 hours) and temperature-controlled environment (21.5–22.5 °C). The animals and procedures used were approved by the University of Texas Southwestern Medical Center at Dallas Institutional Animal Care and Use Committees.
Study design and procedure
Mice were placed on high-fat diet (42% kcal from fat; Harlan Teklad, Madison, WI, USA; stock# 88137) from weaning until they reach the appropriate preoperative weight of 45–50 g, which typically occurs by 16–18 weeks of age. Obese mice were divided into groups of 5–6 and underwent either RYGB or sham surgery, or were not operated. Owing to the complications of the surgery, two sham mice showed overt signs of illness and were removed from the study. RYGB was performed as described previously.5 Briefly, after an overnight fast, animals were maintained under general anesthesia throughout surgery using isoflurane 1–4%, titrated to effect. Using standard sterile techniques, the gut was reconstructed using a Roux-en-Y configuration, such that the combined lengths of the biliopancreatic and Roux limbs comprised 25% of the entire length of gastrointestinal tract. Primary anastomosis was achieved via running suture. Exclusion of the distal stomach remnant and proximal intestine was achieved using a vascular hemostasis clip applied from the greater curvature of the stomach to the lesser curvature between the forestomach and glandular stomach, taking care to preserve the left gastric artery and the abdominal vagus nerve. The sham operation involved a mid-line laparotomy, dissection of the stomach from its ligamentous attachments, complete transection of the intestine, gastrotomy and primary repair (at the analogous sites as occuring during the RYGB procedure). Total anesthesia time was normalized between surgical groups. A liquid diet (elemental) was provided on post surgery day 3 and advanced as tolerated in all mice (including non-operated ones). Solid diet was re-introduced between postoperative days 7 and 8 as tolerated. Animals were killed between postoperative weeks 4 and 5. The percentage of body weight change over the considered postoperative period and number of study animals in each group (n) was as follows: (1) 3.0±3.1% in non-operated animals (n=6), (2) −17.0±1.9% in sham animals (n=4), (3) −28.1±3.8% in RYGB animals (n=5).
Histology
On the day of killing, between 0800–1000 hours, mice were deeply anesthetized with chloral hydrate (500 mg kg−1, intraperitoneal) and transcardially perfused with 10% formalin. Tissues of interest were collected using a dissecting microscope including liver, pancreas and gastrointestinal tract. After perfusion, the muscle layers of the gastrointestinal tract were prepared as sets of whole mounts by separation of the mucosal layer, and flattened on gelatin-coated slides. The liver and pancreas were cut (16 μm) with a cryostat and collected on SuperFrost slides after an overnight incubation in 20% sucrose. All our samples were coverslipped with Vectashield mounting medium. The tdTomato-protein-native fluorescence was directly visualized in our samples. A total of two controls and two vagotomized (bilateral subdiaphragmatic) Phox2b-Cre-tdTomato mice fed on chow (Harlan Teklad 2916) were used for our initial anatomical survey (Figure 1). A total of 14 animals fed on high-fat diet were used for the surgery experiment (Figure 2).
Labeling of the vagus nerve in Phox2b-Cre-tdTomato mice. Schematic diagram of Cre-expressing and fluorescently labeled neurons (black) in the Phox2b-Cre-tdTomato model (a). The expression of tdTomato permits the visualization of vagal neurons in the dorsal motor nucleus of the vagus (brainstem slice) (b, c) and nodose ganglion (cryostat section) (d). Of note, the fluorescence is stable and bright enough to be visible in fixed whole mount and tissue sections without the need of immunohistochemical processing. The tdTomato protein is transported in vagal fibers to their peripheral targets including, among other examples, to the gastric wall where the entire vagal innervation to the myenteric plexus and muscularis was labeled (e). However, vagal sensory and motor, and enteric were intermingled and, therefore, not necessarily distinguishable. In addition to vagal neurons, a subset of enteric neurons in the myenteric plexus is labeled (f). After vagotomy, vagal fibers are eliminated from the myenteric plexus but enteric neurons and their immediate projections are still visible (g). Sparse innervation was found in the gallbladder wall (h), pancreas (i), and hepatic vasculature (j). Scale: 1 mm in (b), 500 μm in (c), 50 μm in (d), 100 μm in (e), 50 μm in (f) and applies to (g), 50 μm in (h), 20 μm in (i) and applies to (j). AP, area postrema; DMV, dorsal motor nucleus of the vagus; MN, myenteric neuron; MP, myenteric plexus, m, muscularis; MR, mechanoreceptors; NG, nodose ganglion; NTS, nucleus of the solitary tract; pv, portal vein; VGX, vagotomy; X, vagus nerve.
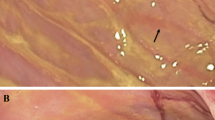
Qualitative and quantitative changes after RYGB in Phox2b-Cre-tdTomato mice. (a) Table summarizing relative changes in the amount of vagal innervation in different gut segments and glucostatic organs in non-operated, sham and RYGB animals. The relative density of fiber innervation based on the visual survey of the tissue was categorized using the following density scale: +++, high density; ++, moderate density; +, low density; +/−, inconsistent or absence of fluorescence. (b) Quantification of vagal supply to the myenteric plexus in non-operated, sham and RYGB animals. Innervation to both proximal and distal stomach is significantly reduced in RYGB mice compared with other groups. (c–j) Representative images of the gastrointestinal wall (whole mounts) in different segments of the gut. Briefly, a significant reduction of the vagal innervation to the stomach, as well as signs of neurodegeneration were noticed after RYGB. However, the vagal innervation to other segments and glucostatic organs was largely intact. Scale: 50 μm in (c) and applies through (f), 100 μm in (j). m, muscularis; n/a, non-applicable; ***P<0.05 by one-way Anova, followed by Bonferroni test. Other abbreviations are indicated in (a).
Microscopy and image analysis
Tissue was processed in standardized conditions and images of whole mounts and sections were generated using a connected scanning stage of a Zeiss microscope (Imager ZI) attached to the ApoTome module. Images were obtained at × 10 or × 20 magnification and automatically captured with a Axiocam MRm digital camera and then stitched together with Axiovision 4.5 (Carl Zeiss, Oberkochen, Germany). The relative abundance of fluorescently labeled neuronal fibers in the myenteric plexus, liver, gallbladder and pancreas was evaluated by considering the number of tdTomato-containing bundles or fibers, using the following density scale: +++, high density; ++, moderate density; +, low density; +/−, inconsistent or absence of fluorescence. In addition, the vagal supply to the myenteric plexus was quantified using Axiovision 4.5. This was done by using a grid of six equidistant lines that spanned the entire width of a 10x image and adding the number and length (μm) of crossings that occurred between labeled myenteric fascicles and the grid. Adobe illustrator CS2 and Adobe Photoshop CS2 were used to combine drawings and digital images into plates. The contrast and brightness of microscopy images were adjusted when necessary.
Results
Labeling of the vagus nerve in Phox2b-Cre-tdTomato mice
The current study employed a unique transgenic model to visualize all vagal neurons in their entirety (Figure 1a). Briefly, Phox2b-Cre-tdTomato mice showed strong endogenous fluorescence in vagal motor and sensory neurons located in the dorsal motor nucleus of the vagus and nodose ganglion, respectively (Figures 1b–d). The peripheral terminals of the above neurons supplying the gastrointestinal tract were also clearly labeled (Figure 1e). This included preganglionic fibers traveling in the myenteric plexus and terminating on myenteric neurons (Figures 1e and f). Of note, the varied mechanoreceptors present in the muscularis were not easily distinguished from vagal motor and enteric fibers, as all of the above fibers were intermingled. In addition, a small subset of postganglionic enteric neurons (∼20%) displayed Cre activity (Figures 1e and f). After a bilateral subdiaphragmatic vagotomy, only tdTomato-containing enteric neurons remained visible, whereas all preganglionic and sensory vagal fibers were eliminated (Figure 1g). Sparse vagal fibers were also found in the pancreas, around large arteries and interlobular postganglionic neurons and islets, and in the hepatic vasculature and gallbladder wall (Figures 1h–j). The innervation to glucostatic organs was lost after subdiaphragmatic vagotomy (not shown). In summary, using the Phox2b-Cre-tdTomato mouse, it is possible to estimate the overall pattern of vagal innervation to the gastrointestinal tract and glucostatic organs. Once again, there are no tracing or immunohistochemical techniques to visualize vagal neurons that are compatible with bariatric surgery and, therefore, the genetic approach used here is indispensable to the success of this study.
Qualitative and quantitative changes after RYGB
A considerable loss of innervation occurred at all sites of surgical manipulation including at the gastrojenunostomy, jejunojejunostomy and adjacent to the site of gastric clipping on both the proximal and distal stomach (Figures 2a and b). Adjacent to the above sites of surgery, the normal appearance of the myenteric plexus was often disrupted, and free-ending bundles of fibers were found in the muscularis compared with the normal appearance of their anatomical equivalents in sham and non-operated animals (Figures 2c–f). In sham animals, whereas a small reduction of innervation was apparent in the stomach adjacent to the site of gastrotomy and repair, as well as at the site of jejunostomy and repair, most of the remainder of gastrointestinal innervation was intact (Figures 2a and g). In RYGB mice, changes in innervation were not apparent in other gut segments (Figures 2a) or in the liver, gallbladder or pancreas (Figure 2a). Lastly, morphological anomalies of vagal fibers were frequently observed in the myenteric plexus and muscularis of RYGB animals, most predominantly in the stomach (Figure 2j). This included dystrophic preganglionic endings circling around myenteric neurons, and extremely swollen axons and terminals in the muscularis and adjacent nerve bundles (Figure 2j).
Discussion
Different branches of the vagus nerve innervate the stomach, intestines and glucostatic organs.10 These branches have an important role in the regulation of food intake, glucose homeostasis and gastrointestinal motility.11 Using a unique reporter system, the current study demonstrated for the first time that RYGB in mice is associated with both changes in the amount of vagal innervation traveling the myenteric plexus and muscularis of the stomach, as well as changes in the morphology of their terminal endings. Our findings are discussed below in the light of known therapeutic effects and complications of RYGB.
Broadly speaking, modified integrity of the vagus nerve may contribute to the many gut adaptations occurring after RYGB. For example, the observed loss of vagal gastric innervation could potentially underlie the gastric stasis and/or dumping syndrome that sometimes occur after RYGB,6 as well as some improvement in gastroesophageal reflux disease.12 The secretion of certain appetite-regulating peptides produced in the stomach is also under the control of autonomic nerves.13 In addition, both gastric vagotomy and vagal inhibition have been reported to result in moderate weight loss in humans14, 15 and, consequently, loss of gastric innervation following RYGB could be a contributing factor to its antiobesity actions.
A limitation of the applicability of our model to humans is the manner of gastric division, which is most analogous to RYGB as performed in humans via open laparotomy where the stomach is often partitioned, but not divided. While the stomach is completely transected during laparoscopic RYGB, short and long-term efficacies after open, non-partitioning RYGB and laparoscopic RYGB are equivalent. Importantly, our model of RYGB faithfully recapitulates effects of the human procedure on body mass, body composition, fecal energy losses, glucose homeostasis, and lipid homeostasis in mice with diet-induced obesity, validating both our model and methodology for studying vagal integrity post-RYGB. In addition, the extent of gastric vagal denervation observed post-RYGB was comparable to that occurring in mice after subdiaphragmatic vagotomy. Thus, no additional vagal denervation would be expected to occur after complete gastric wall transection, obviating the need to control for our method of gastric partitioning.
By contrast, the vagal innervation to the rest of the intestines (celiac branch) and glucostatic organs (hepatic and gastroduodenal branches) appeared largely intact. This is an important observation, as subsets of vagal neurons that supply glucostatic organs have been implicated in the antidiabetic actions of bariatric surgery.3, 4, 5 In addition, an intact para-esophageal branch of the vagus, rather than its hepatic branch,16 is necessary for weight loss following RYGB.2 In summary, our anatomical findings serve as a guideline for further studies directed at elucidating the physiological role of gut–brain communication in bariatric surgery.
References
Buchwald H, Williams SE . Bariatric surgery worldwide 2003. Obes Surg 2004; 14: 1157–1164.
Bueter M, Lowenstein C, Ashrafian H, Hillebrand J, Bloom SR, Olbers T et al. Vagal sparing surgical technique but not stoma size affects body weight loss in rodent model of gastric bypass. Obes Surg 2010; 20: 616–622.
Troy S, Soty M, Ribeiro L, Laval L, Migrenne S, Fioramonti X et al. Intestinal gluconeogenesis is a key factor for early metabolic changes after gastric bypass but not after gastric lap-band in mice. Cell Metab 2008; 8: 201–211.
Breen DM, Rasmussen BA, Kokorovic A, Wang R, Cheung GW, Lam TK . Jejunal nutrient sensing is required for duodenal-jejunal bypass surgery to rapidly lower glucose concentrations in uncontrolled diabetes. Nat Med 2012; 18: 950–955.
Zechner JF, Mirshahi UL, Satapati S, Berglund ED, Rossi J, Scott MM et al. Weight-independent effects of Roux-en-Y gastric bypass on glucose homeostasis via melanocortin-4 receptors in mice and humans. Gastroenterology 2012; 144: 580–590.
Yamada H, Kojima K, Inokuchi M, Kawano T, Sugihara K . Efficacy of celiac branch preservation in Roux-en-y reconstruction after laparoscopy-assisted distal gastrectomy. Surgery 2011; 149: 22–28.
Berthoud HR, Shin AC, Zheng H . Obesity surgery and gut-brain communication. Physiol Behav 2011; 105: 106–119.
Nestoridi E, Kvas S, Kucharczyk J, Stylopoulos N. . Resting energy expenditure and energetic cost of feeding are augmented after Roux-en-Y gastric bypass in obese mice. Endocrinology 2012; 153: 2234–2244.
Scott MM, Williams KW, Rossi J, Lee CE, Elmquist JK. . Leptin receptor expression in hindbrain Glp-1 neurons regulates food intake and energy balance in mice. J Clin Invest 2011; 121: 2413–2421.
Wang FB, Powley TL . Vagal innervation of intestines: afferent pathways mapped with new en bloc horseradish peroxidase adaptation. Cell Tissue Res 2007; 329: 221–230.
Berthoud HR . The vagus nerve, food intake and obesity. Regul Pept 2008; 149: 15–25.
Ardila-Hani A, Soffer EE . Review article: the impact of bariatric surgery on gastrointestinal motility. Aliment Pharmacol Ther 2011; 34: 825–831.
Zhang T, Uchida T, Gomez G, Lluis F, Thompson JC, Greeley GH Jr . Neural regulation of peptide YY secretion. Regul Pept 1993; 48: 321–328.
Mizrahi M, Ya'acov AB, Ilan Y . Gastric stimulation for weight loss. World J Gastroenterol 2012; 18: 2309–2319.
Kral JG . Vagotomy for treatment of severe obesity. Lancet 1978; 1: 307–308.
Shin AC, Zheng H, Berthoud HR . Vagal innervation of the hepatic portal vein and liver is not necessary for Roux-en-Y gastric bypass surgery-induced hypophagia, weight loss, and hypermetabolism. Ann Surg 2012; 255: 294–301.
Acknowledgements
We are grateful to Matthew Harper for his technical assistance and Joel K Elmquist (UT Southwestern Medical Center at Dallas) for his comments on the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Gautron, L., Zechner, J. & Aguirre, V. Vagal innervation patterns following Roux-en-Y gastric bypass in the mouse. Int J Obes 37, 1603–1607 (2013). https://doi.org/10.1038/ijo.2013.48
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ijo.2013.48
Keywords
This article is cited by
-
Novel Insights into the Physiology of Nutrient Sensing and Gut-Brain Communication in Surgical and Experimental Obesity Therapy
Obesity Surgery (2023)
-
Shortened-Interval Dual-Session EDGE Reduces the Risk of LAMS Dislodgement While Facilitating Timely ERCP
Digestive Diseases and Sciences (2021)
-
Surgical Mouse Models of Vertical Sleeve Gastrectomy and Roux-en Y Gastric Bypass: a Review
Obesity Surgery (2019)
-
Gut vagal sensory signaling regulates hippocampus function through multi-order pathways
Nature Communications (2018)
-
Signalling from the periphery to the brain that regulates energy homeostasis
Nature Reviews Neuroscience (2018)