Abstract
Objective:
To prospectively evaluate the effect of weight loss after bariatric surgery on microvascular function in morbidly obese patients with and without metabolic syndrome (MetS).
Methods:
A cohort of morbidly obese patients with and without MetS was studied before surgery and after 12 months of surgery. Healthy lean controls were also examined. Microvascular function was assessed by postocclusive reactive hyperemia (PORH) at forearm skin evaluated by laser Doppler flowmetry (LDF). Cutaneous vascular conductance (CVC) was calculated from laser-Doppler skin blood flow and blood pressure. Regression analysis was performed to assess the contribution of different clinical, metabolic and biochemical parameters to microvascular function.
Results:
Before surgery, 62 obese patients, 39 with MetS and 23 without MetS, and 30 lean control subjects were analyzed. The absolute area under the hyperemic curve (AUCH) CVC of PORH was significantly decreased in obese patients compared with lean control subjects. One year after surgery, AUCH CVC significantly increased in patients free of MetS, including patients that had MetS before surgery. In contrast, AUCH CVC did not significantly change in patients in whom MetS persisted after surgery. Stepwise multivariate regression analysis showed that only changes in HDL cholesterol (HDL-C) and oxidized LDL (oxLDL) independently predicted improvement of AUCH after surgery. These two variables together accounted for 40.9% of the variability of change in AUCH CVC after surgery.
Conclusions:
Bariatric surgery could significantly improve microvascular dysfunction in obese patients, but only in patients free of MetS after surgery. Improvement of microvascular dysfunction is strictly associated to postoperative increase in HDL-C levels and decrease in oxLDL levels.
Similar content being viewed by others
Introduction
Obesity is characterized by impaired microvascular and macrovascular function that may contribute to increased risk of cardiovascular disease.1 Clinical and experimental evidence suggest that microvascular dysfunction is a potential factor explaining the association among obesity, hypertension and insulin resistance, which are major cardiovascular risk factors.2,3 Several methods are used to assess microvascular function in clinical research.4 Postocclusive reactive hyperemia (PORH) at forearm skin evaluated by laser Doppler flowmetry (LDF) has been widely used to assess microvascular function due to its non-invasive nature. PORH is the sudden rise in skin blood flow after release of a brief arterial occlusion and provides an overall measurement of microvascular function.5 However, concerns about the reproducibility of this method have been recently raised.5 In particular, reproducibility studies of LDF in obese patients are scarce.
Bariatric surgery has emerged as an effective treatment for morbid obesity on the basis of both its efficacy6 and beneficial effects on obesity-related comorbidities and total mortality.7, 8, 9 It has been previously shown that bariatric surgery could reverse microvascular dysfunction in obese patients although the determinants that mediate this improvement in microvascular function have not been identified.10, 11, 12, 13, 14 Bariatric surgery-induced weight loss also ameliorates the metabolic syndrome (MetS), a group of clinical manifestations that includes obesity, hypertension, insulin resistance and dyslipidemia.15 Interestingly, the association between surgically induced improvement in microvascular function and metabolic syndrome has not been previously investigated.
The purpose of this study was to investigate the effect of surgically induced weight loss on microvascular function in morbidly obese patients with and without MetS. More specifically, we wanted to determine whether changes in microvascular function after bariatric surgery are associated to resolution of MetS. To this end, we prospectively evaluated microvascular function by LDF in morbidly obese patients with and without MetS before and 12 months after bariatric surgery. We also assessed the contribution of different clinical, metabolic and biochemical parameters to surgically induced improvement in microvascular function.
Materials and methods
Study design and subjects
Obese subjects were recruited from the waiting list for bariatric surgery of the Surgery Unit at Hospital Universitario Virgen del Rocío from November 2009 to March 2011. All obese patients were required to meet NIH guidelines for eligibility for bariatric surgery: BMI ⩾40 kg m−2 or ⩾35 kg m−2 with comorbidities (that is, diabetes, hypertension, dilated cardiomyopathy or sleep apnea).16 The inclusion criteria were male and female patients aged 16–65 years, and agreement to participate in the study by providing a signed consent form. The patients with arterial hypertension, diabetes, cardiomyopathy and sleep apnea were under medical treatment for these obesity complications at the time of the evaluation. Exclusion criteria included acute or chronic inflammatory disease, malignant disease, asthma or any history of alcohol or drug abuse. The experimental protocol was approved by the Ethical Committee of the Hospital Universitario Virgen del Rocío. All participants provided written informed consent to participate in the study. Additional written informed consent was obtained after the surgical procedure.
Obese patients were grouped as patients with MetS or without MetS on the basis of the definition of MetS proposed by the Third Report of the National Cholesterol Education Program Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults Panel III.17 Patients fulfilling three or more of the following criteria were considered as having MetS: (1) central obesity (waist circumference >102 cm in men or >88 cm in women); (2) high blood pressure of 130/85 mm Hg or greater or use of antihypertensive therapy; (3) high fasting glucose (⩾110 mg dl−1); (4) hypertriglyceridemia (⩾150 mg dl−1) and (5) low high-density lipoprotein cholesterol (HDLc) (<40 mg dl−1 for males or <50 mg dl−1 for females). Each subject made a visit at baseline and 12 months after the bariatric surgery. For the 12-month follow-up study, patients in the group with MetS before surgery were further subdivided into two subgroups: patients in whom MetS resolved after surgery and patients in whom MetS persisted after surgery. Healthy lean control subjects were also recruited as a comparison group for the evaluations before surgery.
Clinical and biochemical measurements
Clinical and biochemical measurements were performed before surgery (two weeks earlier) and 12 months after surgery. Every measurement was performed after an overnight fast of 10 h. Systolic and diastolic blood pressure was measured right before LDF evaluation. The values were the mean of two measurements with subjects in sitting position. Measures of weight, height, and waist and hip circumferences were also obtained. Blood samples were drawn from an antecubital vein. Plasma glucose, total serum cholesterol, high-density lipoprotein and triglycerides were measured using a Cobas C chemistry analyzer (Roche Diagnostics, Mannheim, Germany). The Friedewald equation was used to calculate LDL cholesterol (LDLc) from total serum cholesterol, HDLc and triglycerides.18 Plasma insulin was measured by electrical chemiluminescence immunoassay using an ElecsysE170 (Roche Diagnostics, Mannheim, Germany). Hemoglobin A1c (HbA1c) was measured by high-pressure liquid chromatography using a Variant II analyzer (BioRad Laboratories, Hercules, CA, USA) The index of insulin resistance (HOMA) was calculated using the formula: glucose (mmol l−1) × insulin (μU ml−1)/22.5. Plasminogen activator inhibitor-1 (PAI-1) concentrations were determined by enzyme-linked immunosorbent assay (American Diagnostica Inc., Stamford, CT, USA). Ultrasensitive C-reactive protein (CRP) was measured with the CardioPhase hsCRPkit (Dade Behring, Marburg, Germany), the intra and interassay CVs being 2.8 and 4.6%, respectively. Oxidized low-density lipoprotein (oxLDL) was measured with the ELISA kit (Immunodiagnostic Systems, Boldon, UK). The intra and interassay CV was 3.9 and 9%, respectively.
Post-occlusive forearm skin reactive hyperemia (PORH) measurements
Studies were performed in the morning in a quiet, temperature-controlled room (22–24 °C). Subjects were asked to avoid smoking and caffeine- and alcohol-containing drinks for 24 h, and from performing vigorous exercises for at least 12 h before the test. Measurements were taken with subjects in a supine position. On the preoperative study, LDF test was performed two weeks before the surgery.
Changes in cutaneous blood flow (flux) was measured by a commercial single-point laser Doppler flowmetry device (Periflux 5000; bandwidth of 15 kHz, Perimed AB, Järfälla, Sweden) with a thermostatic laser Doppler probe (Probe 481-1, Perimed AB, Järfälla, Sweden). The probe has a fiber separation of 0.25 mm and collects perfusion data at a depth of about 0.5–1 mm. Blood flow data were recorded continuously at a sample rate of 40 recordings per second. Data from the laser Doppler perfusion monitor were analyzed using PeriSoft for Windows, version 2.5.5 (Perimed AB, Järfälla, Sweden). Data files were processed for conversion from mV to PU (perfusion units) by division with the gain factor of the instrument (10 mV PU−1). The laser Doppler probe was placed on the volar surface of the right forearm, 10 cm proximal to the wrist. This position was marked so that exactly the same site was used in all measurements. After a baseline measurement of 3 min, the brachial artery was occluded using a pressure cuff placed around the right upper arm that was inflated to 220 mm Hg. Inflating the cuff took less than 5 s. This local ischemia was held for 4 min, and then deflated. Deflating the cuff was practically instantaneous (<40 mm Hg within 0.2 s). The flux recording was continued for at least 5 min (until the signal reaches the baseline flux). Five different PORH parameters were analyzed (Supplementary Figure 1). The value of skin flux at baseline is defined as the average value of the 3-minute baseline period before occlusion. Maximum response (PORHmax) was defined as the maximum absolute change (PORHpeak) from baseline.19 The area under the hyperemic curve (AUCH) was calculated from the time the cuff was released until the end of the measurement. The area under the occlusion curve (AUCO) was calculated from the time the occlusion started until the end of the occlusion. These five parameters were subsequently transformed into cutaneous vascular conductance (CVC), calculated as the flux divided by the mean arterial pressure in mm Hg. Mean arterial pressure was calculated as (2/3(diastolic blood pressure) + 1/3(systolic blood pressure)). To determine the reproducibility of laser Doppler-derived parameters in the measurement of PORH in obese patients, a study of reproducibility was performed before and after bariatric surgery. Specifically, we investigated within-subject reproducibility of the PORH parameters, that is, reproducibility and variability between measurements. These measurements were performed at different times: (0, 15 min and 24 h after the first measurement). For comparison among groups, we used data from measurements at time 0. All measurements were performed by the same investigator.
Statistical methods
Results are shown as the mean±s.d. or median and interquartile range unless otherwise noted. Between-group differences in normally distributed data were assessed by one-way analysis of variance (ANOVA) followed by the Fisher’s multiple comparison tests to identify differences between groups. For non-normally distributed data, comparisons between groups were analyzed by the Kruskal–Wallis test with Dunn’s multiple comparison tests. To analyze changes after bariatric surgery, the two-way ANOVA for repeated measures was chosen with post hoc Tukey’s comparisons. The contribution of clinical and biochemical parameters to variation in microvascular function was assessed by multivariate regression analysis. Statistically significant predictors were included in the models with a stepwise procedure, after adjusting for age and gender, and antihypertensive and diabetic treatment. Two-sided P-values were assessed for all models. Only variables that had a P<0.05 were included in the final model. To determine the repeatability of LDF, the intraclass correlation coefficients were calculated. Values of intraclass correlation coefficient more than 0.80 were considered excellent agreements.20 To examine the intraday and interday reproducibility of each LDF parameter, within subject coefficients of variation (CVs) were calculated as previously described.21 CVs below 35% were considered acceptable.22 Studies were also compared pairwise to check the precision of the method, the existence of magnitude-dependent bias and systematic error by using the Bland & Altman plots. Statistical analyses were performed with the SPSS statistical package (version 17.0; SPSS, Chicago, IL, USA). Power calculation indicated that our sample size provided an 80% power to detect differences in the vascular reactivity with an effect size as low as 0.2 (Cohen’s f), and 90% power to detect an effect size as low as 0.23, on the basis of two-sided tests at the 0.05 significance level. Power calculations were conducted using GPower3.23
Results
Before analyzing the impact of the MetS on microvascular function, we wanted to determine the reproducibility of the different LDF parameters in obese patients. Intraclass correlation coefficients analysis showed that all LDF measurements were above 0.80 (Supplementary Table 1). As expected, intraday CVs were higher than interday CVs in both evaluations (before and after surgery) (Supplementary Table 2). PORH parameters showed less intraday and interday variation than AUC parameters. Bland-Altman plots displayed no apparent trend or evidence of systematic bias (Supplementary Figures 2 and 3). Thus, all LDF parameters showed a high repeatability with good within-observer reproducibility in our study.
Between-group comparisons in LDF measurements are shown in Table 1. AUCH CVC was significantly decreased in both groups of obese patients compared with lean control subjects. There were no significant differences in this variable between the two groups of obese patients (P=0.958).
A total of 62 patients (53 women; age range 19–65 years) completed the 12-month follow-up assessment. Clinical and metabolic characteristics of the 62 obese patients and 30 lean control subjects are described in Table 2. All groups were matched for age and gender. As expected, there was a high prevalence of diabetes and hypertension among obese patients with MetS. Systolic blood pressure, fasting glucose, HbA1c, total cholesterol, LDLc and triglycerides concentrations were higher in obese patients with MetS compared with both obese subjects without MetS and lean subjects. Diastolic blood pressure, heart rate and fasting insulin were higher in the two obese groups. Furthermore, PAI-1, CRP and oxLDL levels were higher in obese patients when compared with lean control subjects.
One-year follow-up data for all patients are shown in Table 3. A total of 45 and 17 patients underwent laparoscopic sleeve gastrectomy and Roux-en-Y gastric bypass, respectively. No differences in weight loss between the different types of bariatric surgery were observed (P=0.223). The disparity between male and female patients in the current study (85.5% women) is in agreement with studies reporting that women seek bariatric surgery more often than men.24 However, no significant differences were found among the three study groups in terms of gender and type of surgery (P=0.798 and P=0.963, respectively). Resolution of MetS was observed in 27 patients. Bariatric surgery reduced significantly anthropometric values in all patients. However, patients with MetS after surgery displayed higher BMI than patients without MetS. Higher waist and hip circumference was observed in patients with MetS after surgery compared with patients in whom MetS was resolved after surgery. Bariatric surgery resulted in a decrease in DBP and heart rate in patients in whom MetS was resolved after surgery. All patients reduced fasting insulin, HOMA-IR, HbA1c, PAI-1 and CRP levels after surgery. A significant improvement in fasting glucose, total cholesterol, LDLc and HDLc, triglycerides and oxLDL was observed in patients free of MetS but not in patients with MetS after surgery. Comparison between groups showed elevated fasting glucose, fasting insulin, HOMA-IR, HbA1c, triglycerides, CRP and oxLDL levels in the group of patients in whom MetS persisted after surgery compared with the other groups of obese patients. Low HDL cholesterol was also observed on these patients compared with the other groups.
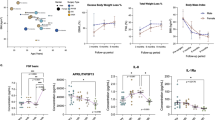
Bariatric surgery resulted in a significant increase in AUCH CVC in patients free of MetS, including patients that had MetS before surgery (P<0.05) (Figure 1). In contrast, AUCH CVC did not significantly change in patients in whom MetS was not resolved after surgery (P=0.72). To identify determinants of microvascular function changes after surgery, correlation analyses were performed between all clinical and biochemical variables and AUCH CVC. Increased AUCH CVC after bariatric surgery was significantly associated with an increase in HDLc (Pearson’s R=0.53, P<0.001) and a decrease in oxLDL (R=−0.54, P<0.001), fasting glucose (R=−0.27, P=0.04) and HbA1c (R=−0.29, P=0.03). When these biochemical variables were entered into a stepwise multivariate regression analysis, only changes in HDLc and oxLDL concentrations independently predicted improvement of AUCH CVC after surgery (Table 4). These two variables together accounted for 40.9% of the variability of change in AUCH CVC after surgery. Interestingly, HDLc levels correlated significantly with oxLDL (R=−0.30, P=0.03).
AUCH CVC measurements in obese patients before and 1 year after bariatric surgery. Patients were classified in three groups: patients without MetS before surgery (MetS−/MetS−), patients in whom MetS had been resolved after bariatric surgery (MetS+/MetS−), and patients in whom MetS persisted after bariatric surgery (MetS+/MetS−). Data are presented as mean and s.d. **P<0.01; ***P<0.001 (Tukey’s test; within-subject comparison).
Discussion
Our prospective study reveals an association between MetS and surgically induced improvement of microvascular dysfunction in obese patients. Thus, our results are in agreement with studies showing a functional association between microvascular dysfunction and metabolic syndrome.25 Indeed, clinical and experimental evidence suggests that microvascular dysfunction may have a central role in the development of obesity-associated hypertension and insulin resistance.3,26
To assess microvascular function, we utilized PORH at forearm skin evaluated by LDF, a method widely used in vascular research.5 PORH has been shown to be fairly reproducible in lean subjects.27, 28, 29 Our reproducibility analysis extends these results demonstrating that all LDF parameters of our study are reproducible both intraday (within-day) and interday (between-day) in obese patients. The reproducibility of PORH parameters in our study was higher than those reported in other studies in non-obese subjects.30 To this regard, it should be noted that in our study the position of laser probe was marked so that exactly the same site was used in all measurements. This was possible due to the short period of time (24 h) between measurements, another difference between our study and previous studies30 that could explain our relatively higher reproducibility values. Several different parameters can be obtained when performing PORH.5 AUCH is a commonly used parameter that simultaneously measures velocity, intensity and duration of the hyperemia response.5 Several studies have demonstrated that AUCH is a reliable indicator of microvascular dysfunction in patients at risk of cardiovascular disease.31, 32, 33, 34 Our LDF studies performed before surgery revealed that AUCH was significantly decreased in obese patients compared with lean control subjects. These results add to the growing body of evidence that microvascular function is impaired in obese patients.1,2
Surgically induced weight loss resulted in a significant improvement in microvascular function in obese patients without MetS, including patients that had MetS before surgery. These results are in agreement with previous studies showing that microvascular dysfunction could be reversed in obese patients after successful bariatric surgery.10, 11, 12, 13, 14 However, our results reveal that obese patients with MetS after surgery, despite significant weight loss, still exhibit microvascular dysfunction. These findings suggest that MetS is a strong determinant of improvement of microvascular function associated with surgically induced weight loss. Stepwise multiple linear regression analysis revealed that low HDLc levels is the component of MetS that best predicts lack of improvement in microvascular dysfunction in obese patients.
In addition to HDLc, our regression analysis identified oxLDL as an independent predictor of improvement of microvascular function in obese patients. An increase in HDLc levels and a decrease in oxLDL levels were independently associated with improvement of microvascular function after surgery. The relationship between HDLc, oxLDL and AUCH CVC can, at least in part, explain the lack of improvement in microvascular function in patients in whom MetS persisted after surgery given that HDLc and oxLDL levels did not significantly change in this group after surgery. Although HDLc and oxLDL are known to have an important role in endothelial function,35,36 the contribution of these factors to obesity-related microvascular dysfunction has been less explored. Our results indicate that HDLc and oxLDL are good predictors of improvement in microvascular function in obese patients after surgery.
Our findings that surgically induced weight loss leads to increased HDLc levels and decreased oxLDL levels are in agreement with previous studies.36,37 Our study further extends these findings showing that HDLc levels correlate negatively with oxLDL levels in patients after bariatric surgery. These results provide a potential mechanism by which surgically induced weight loss might improve microvascular dysfunction in obesity. Experimental data obtained in human subjects38 and in animal models39 have demonstrated that HDL can counteract the inhibitory effect of oxLDL on vascular reactivity. Thus, it is tempting to speculate that bariatric surgery might induce an increase in HDLc levels leading to a reduction in oxidation of LDL and, in turn, an improvement of microvascular function. However, it is important to note that not only the levels but also the functional capacity of HDLc is important for endothelial function.40 To this regard, a recent study has shown that bariatric surgery in obese adolescents increased HDL levels but not functional capacity (determined as its ability to stimulate endothelial nitric oxide synthase activation in vitro).41 Thus, it would be important in future studies to analyze the relationship between HDL functionality and surgically induced improvement in microvascular function.
Why the patients in whom MetS persisted after surgery did not show an improvement in microvascular function remains to be determined. It should be noted that this group of patients, despite significant weight loss after surgery, still remains morbidly obese. Although this might suggest that the lack of microvascular improvement in this group of patients is due to insufficient weight loss, no independent association was found between the degree of surgically induced weight loss and microvascular function in the whole set of obese patients. Nevertheless, an indirect effect of weight loss in microvascular function through changes in HDLc and oxLDL levels cannot be ruled out.
The main limitation of our study is that we cannot conclude whether the surgically induced improvement in microvascular function in obese patients is endothelium-dependent. The major mediators contributing to PORH in the skin include sensory nerves and endothelium-derived hyperpolarizing factors.4 In this regard, a recent study has shown that cytochrome epoxygenase metabolites have a major role in skin PORH.42 It would be interesting to determine the contribution of these pathways in surgically induced microvascular improvement in obese patients.
In summary, the present study shows that bariatric surgery could significantly improve microvascular dysfunction in obese patients, but only in those patients free of MetS after surgery. This improvement is strictly associated to postoperative increase in HDLc levels and decrease in oxLDL levels. Our results suggest that HDLc and oxLDL are good markers of improvement of microvascular function associated with surgically induced weight loss.
References
Stapleton PA, James ME, Goodwill AG, Frisbee JC . Obesity and vascular dysfunction. Pathophysiology 2008; 15: 79–89.
de Jongh RT, Serne EH, IJ RG, de Vries G, Stehouwer CD . Impaired microvascular function in obesity: implications for obesity-associated microangiopathy, hypertension, and insulin resistance. Circulation 2004; 109: 2529–2535.
De Boer MP, Meijer RI, Wijnstok NJ, Jonk AM, Houben AJ, Stehouwer CD et al. Microvascular dysfunction: a potential mechanism in the pathogenesis of obesity-associated insulin resistance and hypertension. Microcirculation 2012; 19: 5–18.
Roustit M, Cracowski JL . Assessment of endothelial and neurovascular function in human skin microcirculation. Trends Pharmacol Sci 2013; 34: 373–384.
Roustit M, Cracowski JL . Non-invasive assessment of skin microvascular function in humans: an insight into methods. Microcirculation 2012; 19: 47–64.
Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K et al. Bariatric surgery: a systematic review and meta-analysis. Jama 2004; 292: 1724–1737.
Sjostrom L, Lindroos AK, Peltonen M, Torgerson J, Bouchard C, Carlsson B et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med 2004; 351: 2683–2693.
Sjostrom L, Narbro K, Sjostrom CD, Karason K, Larsson B, Wedel H et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med 2007; 357: 741–752.
Vest AR, Heneghan HM, Agarwal S, Schauer PR, Young JB . Bariatric surgery and cardiovascular outcomes: a systematic review. Heart 2012; 98: 1763–1777.
Rossi M, Nannipieri M, Anselmino M, Pesce M, Muscelli E, Santoro G et al. Skin vasodilator function and vasomotion in patients with morbid obesity: effects of gastric bypass surgery. Obes Surg 2011; 21: 87–94.
Lind L, Zethelius B, Sundbom M, Eden Engstrom B, Karlsson FA . Vasoreactivity is rapidly improved in obese subjects after gastric bypass surgery. Int J Obes (Lond) 2009; 33: 1390–1395.
Williams IL, Chowienczyk PJ, Wheatcroft SB, Patel AG, Sherwood RA, Momin A et al. Endothelial function and weight loss in obese humans. Obes Surg 2005; 15: 1055–1060.
Brethauer SA, Heneghan HM, Eldar S, Gatmaitan P, Huang H, Kashyap S et al. Early effects of gastric bypass on endothelial function, inflammation, and cardiovascular risk in obese patients. Surg Endosc 2011; 25: 2650–2659.
Gokce N, Vita JA, McDonnell M, Forse AR, Istfan N, Stoeckl M et al. Effect of medical and surgical weight loss on endothelial vasomotor function in obese patients. Am J Cardiol 2005; 95: 266–268.
Grundy SM, Brewer HB Jr, Cleeman JI, Smith SC Jr, Lenfant C . Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation 2004; 109: 433–438.
Gastrointestinal surgery for severe obesity: National Institutes of Health Consensus Development Conference Statement. Am J Clin Nutr 1992; 55: 615S–619S.
Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). Jama 2001; 285: 2486–2497.
Friedewald WT, Levy RI, Fredrickson DS . Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972; 18: 499–502.
Jimenez-Morales AI, Ruano J, Delgado-Lista J, Fernandez JM, Camargo A, Lopez-Segura F et al. NOS3 Glu298Asp polymorphism interacts with virgin olive oil phenols to determine the postprandial endothelial function in patients with the metabolic syndrome. J Clin Endocrinol Metab 2011; 96: E1694–E1702.
Fleiss J . The Design and Analysis of Clinical Experiments. John Wiley & Sons: New York, 1986.
Donald AE, Charakida M, Cole TJ, Friberg P, Chowienczyk PJ, Millasseau SC et al. Non-invasive assessment of endothelial function: which technique? J Am Coll Cardiol 2006; 48: 1846–1850.
Harris RA, Padilla J, Hanlon KP, Rink LD, Wallace JP . Reproducibility of the flow-mediated dilation response to acute exercise in overweight men. Ultrasound Med Biol 2007; 33: 1579–1585.
Faul F, Erdfelder E, Lang AG, Buchner A . G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 2007; 39: 175–191.
Samuel I, Mason EE, Renquist KE, Huang YH, Zimmerman MB, Jamal M . Bariatric surgery trends: an 18-year report from the International Bariatric Surgery Registry. Am J Surg 2006; 192: 657–662.
Serne EH, de Jongh RT, Eringa EC, IJ RG, Stehouwer CD . Microvascular dysfunction: a potential pathophysiological role in the metabolic syndrome. Hypertension 2007; 50: 204–211.
Levy BI, Schiffrin EL, Mourad JJ, Agostini D, Vicaut E, Safar ME et al. Impaired tissue perfusion: a pathology common to hypertension, obesity, and diabetes mellitus. Circulation 2008; 118: 968–976.
Tibirica E, Matheus AS, Nunes B, Sperandei S, Gomes MB . Repeatability of the evaluation of systemic microvascular endothelial function using laser doppler perfusion monitoring: clinical and statistical implications. Clinics (Sao Paulo) 2011; 66: 599–605.
Yvonne-Tee GB, Rasool AH, Halim AS, Rahman AR . Reproducibility of different laser Doppler fluximetry parameters of postocclusive reactive hyperemia in human forearm skin. J Pharmacol Toxicol Methods 2005; 52: 286–292.
Agarwal SC, Allen J, Murray A, Purcell IF . Comparative reproducibility of dermal microvascular blood flow changes in response to acetylcholine iontophoresis, hyperthermia and reactive hyperaemia. Physiol Meas 2010; 31: 1–11.
Roustit M, Blaise S, Millet C, Cracowski JL . Reproducibility and methodological issues of skin post-occlusive and thermal hyperemia assessed by single-point laser Doppler flowmetry. Microvasc Res 2010; 79: 102–108.
Stiefel P, Moreno-Luna R, Vallejo-Vaz AJ, Beltran LM, Costa A, Gomez L et al. Which parameter is better to define endothelial dysfunction in a test of postocclusive hyperemia measured by laser-Doppler flowmetry? Coron Artery Dis 2012; 23: 57–61.
Kruger A, Stewart J, Sahityani R, O’Riordan E, Thompson C, Adler S et al. Laser Doppler flowmetry detection of endothelial dysfunction in end-stage renal disease patients: correlation with cardiovascular risk. Kidney Int 2006; 70: 157–164.
Yamamoto-Suganuma R, Aso Y . Relationship between post-occlusive forearm skin reactive hyperaemia and vascular disease in patients with Type 2 diabetes—a novel index for detecting micro- and macrovascular dysfunction using laser Doppler flowmetry. Diabet Med 2009; 26: 83–88.
Rossi M, Bradbury A, Magagna A, Pesce M, Taddei S, Stefanovska A . Investigation of skin vasoreactivity and blood flow oscillations in hypertensive patients: effect of short-term antihypertensive treatment. J Hypertens 2011; 29: 1569–1576.
Galle J, Hansen-Hagge T, Wanner C, Seibold S . Impact of oxidized low density lipoprotein on vascular cells. Atherosclerosis 2006; 185: 219–226.
Tran-Dinh A, Diallo D, Delbosc S, Varela-Perez LM, Dang QB, Lapergue B et al. HDL and endothelial protection. Br J Pharmacol 2013; 169: 493–511.
Garrido-Sanchez L, Garcia-Almeida JM, Garcia-Serrano S, Cardona I, Garcia-Arnes J, Soriguer F et al. Improved carbohydrate metabolism after bariatric surgery raises antioxidized LDL antibody levels in morbidly obese patients. Diabetes Care 2008; 31: 2258–2264.
Persegol L, Verges B, Foissac M, Gambert P, Duvillard L . Inability of HDL from type 2 diabetic patients to counteract the inhibitory effect of oxidised LDL on endothelium-dependent vasorelaxation. Diabetologia 2006; 49: 1380–1386.
Matsuda Y, Hirata K, Inoue N, Suematsu M, Kawashima S, Akita H et al. High density lipoprotein reverses inhibitory effect of oxidized low density lipoprotein on endothelium-dependent arterial relaxation. Circ Res 1993; 72: 1103–1109.
Duffy D, Rader DJ . Update on strategies to increase HDL quantity and function. Nat Rev Cardiol 2009; 6: 455–463.
Matsuo Y, Oberbach A, Till H, Inge TH, Wabitsch M, Moss A et al. Impaired HDL function in obese adolescents: Impact of lifestyle intervention and bariatric surgery. Obesity (Silver Spring) 2013; 21: E687–E695.
Cracowski JL, Gaillard-Bigot F, Cracowski C, Sors C, Roustit M, Millet C . Involvement of cytochrome epoxygenase metabolites in cutaneous postocclusive hyperemia in humans. J Appl Physiol 2013; 114: 245–251.
Acknowledgements
Funding for this study was provided by the Andalusian Regional Ministry of Health (PI-0269/2008). We thank Rocío Infante-Fontán for technical assistance with biochemical analyses. We also thank Francisco J Tinahones and José Manuel Fernández Real for critical review of the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on International Journal of Obesity website
Supplementary information
Rights and permissions
About this article
Cite this article
Martín-Rodríguez, J., Cervera-Barajas, A., Madrazo-Atutxa, A. et al. Effect of bariatric surgery on microvascular dysfunction associated to metabolic syndrome: a 12-month prospective study. Int J Obes 38, 1410–1415 (2014). https://doi.org/10.1038/ijo.2014.15
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ijo.2014.15
Keywords
This article is cited by
-
Gastric Bypass Improves Microvascular Perfusion in Patients with Obesity
Obesity Surgery (2021)
-
Bile acid, glucose, lipid profile, and liver enzyme changes in prediabetic patients 1 year after sleeve gastrectomy
BMC Surgery (2020)
-
Short- and Long-Term Effects of Bariatric Surgery on Vascular Phenotype
Obesity Surgery (2019)
-
Microcirculatory Improvement Induced by Laparoscopic Sleeve Gastrectomy Is Related to Insulin Sensitivity Retrieval
Obesity Surgery (2018)
-
The Effects of Bariatric Surgery-Induced Weight Loss on Adipose Tissue in Morbidly Obese Women Depends on the Initial Metabolic Status
Obesity Surgery (2016)