Abstract
Background:
Bone morphogenetic protein-3b (BMP-3b) is a member of the transforming growth factor-β superfamily and has several activities that differ from those of other BMPs. We previously found that BMP-3b is highly expressed in adipocytes, its level is increased during obesity, and it inhibits adipogenesis by suppressing peroxisome proliferator-activated receptor γ (PPARγ) in vitro. However, the function of BMP-3b in adipose tissues in vivo remains unknown.
Methods:
To determine the role of BMP-3b overexpression in adipose tissues in vivo, we generated transgenic mice (BMP-3b Tg) by using a conditional overexpression approach in fatty acid-binding protein 4-expressing adipocytes. We examined BMP-3b Tg mice fed a high-fat diet to elucidate the effects of BMP-3b on obesity. Adipocyte function was evaluated as expression of adipogenic and lipogenic markers in adipose tissue. We also performed glucose and insulin tolerance tests (GTT and ITT, respectively), and biochemical analysis of serum and measured energy expenditure by indirect calorimetry.
Results:
BMP-3b Tg mice fed a high-fat diet showed decreases in weight gain, fat-pad mass and adipocyte area, compared with wild-type mice. The adipose tissues of BMP-3b Tg mice showed downregulated expression of PPARγ and its target gene encoding fatty acid translocase/CD36. In addition, BMP-3b Tg mice had decreased blood glucose levels on GTT and ITT, and their serum leptin levels were decreased and adiponectin concentrations were increased. These changes in BMP-3b Tg mice were accompanied by increased energy expenditure, indicated as increased locomotor activity and oxygen consumption.
Conclusions:
These results provide in vivo evidence that BMP-3b regulates adipocyte function to cause an anti-obesity effect.
Similar content being viewed by others
Introduction
Adipose tissue plays a critical role in energy balance, and lipid and glucose metabolism and, therefore, contributes to the development of obesity. In addition, adipose tissue is considered to be not only an energy storage site but also an endocrine organ that secretes adipokines. Furthermore, the gain in body weight characteristic of obesity is associated with adipocyte hypertrophy and increased adipose tissue weight, leading to imbalanced secretion of adipokines and the induction of insulin resistance and diabetes.1, 2, 3, 4, 5, 6
Bone morphogenetic proteins (BMPs) are a subgroup of the transforming growth factor-β (TGF-β) superfamily. BMPs have pleiotropic effects in numerous tissues and physiological processes. In adipose tissues, BMP-2 and -4 enhance white adipogenesis, whereas BMP-7 activates brown adipogenesis.7, 8, 9
We previously isolated BMP-3b from rat and human femurs.10, 11 This factor has also been identified as growth/differentiation factor 10 through degenerate PCR screening for TGF-β family molecules in mouse brain and lung.12 Unlike other BMPs (for example, BMP-2 and -4), BMP-3b inhibits osteogenesis and induces head formation in embryos.13, 14 In particular, BMP-3b inhibits osteoblast differentiation through the Smad2/3 pathway by counteracting Smad1/5/8 signaling.15 We also showed that BMP-3b and its receptors (activin receptor-like kinase 4 (ALK-4), and activin type IIA receptor (ActRIIA)) are highly expressed in adipocytes and that the expression of BMP-3b is increased in mice with diet-induced obesity compared with non-obese mice.16 Furthermore, the expression of human BMP-3b is elevated in the adipose tissue of overweight women.17 Our in vitro experiments have shown that the knockdown of BMP-3b enhances adipogenesis, whereas exogenous BMP-3b inhibits the differentiation of cultured adipocytes by suppressing the expression of peroxisome proliferator-activated receptor γ (PPARγ) and CCAAT enhancer-binding protein α (C/EBPα).16 These data suggest that BMP-3b acts as an adipokine to regulate adipocyte function, including adipogenesis.
In the present study, to determine the role of BMP-3b in adipose tissues in vivo, we generated transgenic (Tg) mice in which BMP-3b was conditionally overexpressed in fatty acid-binding protein 4 (FABP4)-overexpressing adipocytes. We report the characteristics of BMP-3b Tg mice, which were resistant to high-fat diet (HFD)-induced obesity through the regulation of adipocyte function including adipogenesis and lipogenesis. In addition, BMP-3b Tg mice showed improved glucose tolerance and insulin sensitivity.
Materials and methods
Animals
All experiments were approved by the Animal Care and Use Committee of the National Cerebral and Cardiovascular Center Research Institute (Osaka, Japan), whose laboratory animal facilities comply with the ‘Basic Policies for the Conduct of Animal Experimentation in the Ministry of Health, Labour, and Welfare’ according to assessment by the Center for Accreditation of Laboratory Animal Care and Use, Japan Health Sciences Foundation, and were performed in accordance with the approved guidelines. C57BL/6 J mice were obtained from CLEA Japan Inc. (Tokyo, Japan). FABP4-Cre mice (B6.Cg-Tg (Fabp4-Cre)1Rev/J) were obtained from the Jackson Laboratory (stock 005069, Bar Harbor, ME, USA). All mice were housed under a 12:12-h light:dark cycle and had unrestricted access to standard diet (STD) (12 kcal% fat, 29 kcal% protein and 59 kcal% carbohydrate; CE-2, CLEA Japan Inc.) or HFD (57 kcal% fat, 20 kcal% protein and 23 kcal% carbohydrate; High-Fat Diet 32, CLEA Japan Inc.). For diet-induced obesity, the mice received HFD beginning at 5 weeks of age.
Generation of BMP-3b Tg mice
The pCAG-CAT-EGFP plasmid, which contained the CAG promoter (a modified chicken β-actin promoter with the CMV-IE enhancer), the CAT gene flanked by directly repeated loxP sequences and EGFP complimentary DNA (cDNA), was kindly provided by Dr Jun-ichi Miyazaki18 (Osaka University, Osaka, Japan). In CAG-CAT-EGFP Tg mice, the CAT gene is driven by the CAG promoter before recombination, whereas the EGFP gene is driven by the same CAG promoter only after Cre-mediated recombination.17 The pCAG-CAT-EGFP plasmid was modified by replacing EGFP with mouse BMP-3b (mBMP-3b) cDNA (Figure 1a). The CAG-CAT-mBMP-3b region was excised from the pCAG-CAT-mBMP-3b plasmid and microinjected into the pronucleus of C57BL/6 J mouse oocytes to produce Tg mice. Founder Tg mice were identified by PCR analysis of tail DNA. The CAG-CAT-mBMP-3b mice constitutively express CAT; upon Cre-mediated recombination, BMP-3b expression is induced, with concomitant loss of CAT expression. To generate Tg mice in which BMP-3b overexpression was restricted to adipose tissues, CAG-CAT-mBMP-3b Tg mice were mated with FABP4-Cre Tg mice; the resulting progeny were genotyped by PCR analysis using a primer set specific for the BMP-3b and Cre genes. The rate of FABP4-Cre-mediated recombination is ~30–60% in various adipose tissues.19 Despite the variable recombination efficiency among different adipose tissues, BMP-3b is constitutively overexpressed in all of them. Unless otherwise indicated, all experiments in this study were performed on male double-Tg mice, in which BMP-3b was inducibly overexpressed in FABP4-expressing adipocytes (referred to as BMP-3b Tg) and their sex-matched wild-type (Wt) littermates. Female and male BMP-3b Tg mice were similar in phenotype (data not shown).
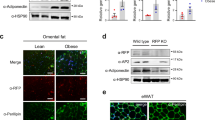
Generation and characterization of transgenic mice with overexpression of BMP-3b in adipose tissues. (a) Generation of transgenic mice for the tissue-specific, Cre-inducible overexpression of BMP-3b in FABP4-expressing adipocytes. To restrict BMP-3b overexpression to adipose tissue, we crossed CAG-CAT-mBMP-3b mice with FABP4-Cre Tg mice. (b) Gene expression of BMP-3b in various adipose tissues in Wt (open bars) and BMP-3b Tg (filled bars) male (left) and female (right) mice (age, 14 weeks; female, n=3; male, n=5). Expression levels were quantified by quantitative real-time PCR analysis. **P<0.01 compared with Wt. (c) The BMP-3b protein levels in various adipose tissues from the female Wt and Tg mice used in the gene expression analysis were analyzed by western blotting. The apparent lack of BMP-3b protein in mesenteric fat likely is due to poor correlation between BMP-3b mRNA levels and the protein content in the investigated adipose tissues; for secreted proteins, such as BMPs, the protein content in a particular tissue represents the end result of gene transcription and protein biosynthesis, secretion and degradation. Size markers are shown at the left of the image; the arrowhead indicates the size of BMP-3b secreted from 3T3-L1 cells. (d) Protein levels of phosphorylated Smad2 (pSmad2) and total Smad2/3 (tSmad2/3) in the perirenal adipose tissues from Wt and Tg mice on HFD (age, 30 weeks, n=5) were analyzed by western blotting. The relative integrated density of each protein band was quantified by using the program Multi Gauge. *P<0.05 compared with Wt. a.u., arbitrary units; BAT, brown adipose tissue; epi, epididymal fat; mes, mesenteric fat; peri, perirenal fat; subQ, subcutaneous fat; WAT, white adipose tissue.
Quantitative real-time PCR analysis
Total RNA was extracted from tissues by using TRIzol (Invitrogen, Life Technologies, Carlsbad, CA, USA) according to the manufacturer’s instructions. Template cDNA was synthesized by using a quantitative cDNA kit (Qiagen, Hamburg, Germany). Quantitative real-time PCR analysis was performed by using SYBR Premix Ex Taq (TaKaRa, Shiga, Japan) and specific primer sets (Supplementary Table 1) in a LightCycler System (Roche, Basel, Switzerland). Gene copy numbers were derived from a standard curve obtained by using a serially diluted-specific plasmid or adipose tissue cDNAs and were normalized against ribosomal protein S18 mRNA (messenger RNA).
Western blotting
Western blotting of immunoprecipitates obtained by using anti-mBMP-3b antibody was performed as described previously.16 Briefly, tissues were homogenized and lysed in lysis buffer (50 mm Tris-HCl (pH 7.4), 150 mm NaCl, 1% Triton X-100) containing protease inhibitor cocktail (catalog no. P8340, Sigma-Aldrich, St Louis, MO, USA). Each adipose tissue mass was harvested in its entirety and processed in a single extraction; each extract was immunoprecipitated by using an anti-mBMP-3b IgG antibody immunoprecipitation as previously described;16 25% of each immunoprecipitate underwent western blot analysis with anti-human BMP-3b antibody (catalog no. AF1543, R&D Systems, Minneapolis, MN, USA).16 BMP-3b protein was not detected in mesenteric fat likely because this fat pad was much smaller than other adipose tissues (for example, 61±8.0 mg for the mesenteric fat pad and 160±2.7 mg for subcutaneous fat in Tg mice, n=3).
For western blotting of Smad2/3 signaling proteins, adipose tissue was lysed in lysis buffer (50 mm Tris-HCl (pH 7.4), 150 mm NaCl, 5 mm EDTA, 1% NP-40, 0.1% SDS) containing protease inhibitor cocktail (catalog no. 04080-11, 07574-61, Nacalai Tesque, Kyoto, Japan). Equal amounts of lysates underwent western blot analysis as described previously15 with anti-Smad2/3 antibody (catalog no. 3102, Cell Signaling Technology, Beverly, MA, USA) and anti-phospho-Smad2 (Ser245/250/255) (catalog no. 3104, Cell Signaling Technology). Signals were detected by using an image quantification system (LAS-4000, GE Healthcare UK Ltd, Little Chalfont, England) and quantified by using Multi Gauge (GE Healthcare UK Ltd.).
Histological analysis
Adipose tissues were fixed in 4% paraformaldehyde, dehydrated in ethanol, embedded in paraffin, cut in sections (thickness, 6 μm), and stained with hematoxylin and eosin. The area of adipocytes in Wt and Tg mice (n=5) was determined. Morphometric analysis to determine adipocyte area was performed by using WinROOF software (Mitani, Fukui, Japan).
Glucose and insulin tolerance tests
Mice on HFD (30 weeks of age) were fasted 16 h before intraperitoneal injection of saline-diluted glucose (1 mg g−1 body weight; Wako Pure Chemical Industries Ltd, Osaka, Japan) or insulin (0.75 mIU g−1 body weight; Sigma-Aldrich). Blood glucose was measured (Accu-Check glucose monitor, Roche) at 0, 15, 30, 60 and 120 min after injection.
Blood biochemistry
Serum leptin, insulin and adiponectin levels were determined by using mouse ELISA kits for leptin (Morinaga, Tokyo, Japan), insulin (Morinaga) and adiponectin (Otsuka Pharmaceutical Co., Tokyo, Japan), respectively. Serum total cholesterol and triglycerides were quantified automatically (Dri-Chem 7000, FujiFilm, Tokyo, Japan). Serum nonesterified fatty acids were measured by using LabAssay NEFA (Wako).
Indirect calorimetry
Wt and BMP-3b Tg mice on HFD (11 weeks of age) were used for simultaneous assessment of the whole-body metabolic profile in an indirect calorimeter (Comprehensive Lab Animal Monitoring System, Columbus Instruments, Columbus, OH, USA). After the mice had adapted to the environment of the calorimetry chambers (at least 3 days), data were recorded continuously every 3–4 days and analyzed. Food intake, O2 consumption (VO2) and CO2 production (VCO2) were measured. Energy expenditure was calculated as VO2 × [3.815+(1.232 × VCO2/VO2)]. Locomotor activity was calculated according to the number of infrared beam breaks, and the respiratory quotient was calculated as VCO2/VO2.
Statistical analysis
Data are expressed as the mean±s.e.m. Statistical significance was assessed by using the Student t-test. A P-value <0.05 was considered statistically significant. Data distribution was assumed to be normal, but this was not formally tested. No animals or data were excluded. No statistical methods were used to predetermine sample sizes. Data collection and analysis were not randomized but performed blind to the conditions of the experiments.
Results
Generation of transgenic mice overexpressing BMP-3b in adipose tissues
To address the physiological role of BMP-3b in various tissues, we established Tg mice in which the expression of BMP-3b was tissue-specific and Cre-inducible (designated CAG-CAT-mBMP-3b mice) (Figure 1a). To restrict BMP-3b overexpression to adipose tissues, we mated CAG-CAT-mBMP-3b mice with FABP4-Cre Tg mice (Figure 1a);18 we designated the mice in which BMP-3b was inducibly overexpressed in FABP4-expressing adipocytes as ‘BMP-3b Tg mice.’ We used quantitative real-time PCR and western blot analysis to confirm tissue-specific gene expression and protein production, respectively, of BMP-3b in several adipose tissues (Figures 1b and c). Among the adipose tissues examined, BMP-3b mRNA levels (copy numbers normalized relative to S18 mRNA) were 10–86 times higher in male BMP-3b Tg mice than in male Wt mice. In addition, BMP-3b mRNA levels in liver were 4–50 times lower than in adipose tissues in both male and female Tg mice, although hepatic BMP-3b levels were higher in Tg mice than in Wt mice (Figure 1b). Because FABP4 is also expressed in macrophages,20 the source of BMP-3b in the liver might be macrophages, which aggregate in the hepatic tissue during obesity. Furthermore, BMP-3b continued to be overexpressed in the adipose tissues of BMP-3b Tg mice fed a HFD (Supplementary Figure 1). The expression level of other BMPs (such as BMP-2, -4 and -7) in most adipose tissues was similar between Wt and Tg mice (Supplementary Figure 2). The levels of BMP-3b protein were higher in the adipose tissues of BMP-3b Tg mice than in those of Wt mice, and the molecular weights of the BMP-3b products in these tissues were identical to those of the protein secreted from 3T3-L1 cells (preadipocyte cell line).16 The two molecular forms of BMP-3b are considered to arise due to differences in processing sites or N-glycosylation.14, 16 Finally, we examined intracellular signaling in BMP-3b Tg mice and found that Smad2 phosphorylation was increased in the adipose tissues (Figure 1d); this finding is consistent with the effects of exogenous BMP-3b in 3T3-L1 cells (data not shown). Together, these data indicate that BMP-3b was overexpressed and functional in the adipose tissues of BMP-3b Tg mice.
BMP-3b Tg mice fed HFD showed decreased weight gain, fat-pad weight and adipocyte area
To examine the effects of BMP-3b overexpression in adipose tissues, we first assessed the body weight of mice fed either a STD or HFD. STD-fed BMP-3b Tg mice weighed slightly but significantly less than did control Wt mice from 5 to 16 weeks of age (Tg at 14 weeks: 25.8±0.36 g, n=16; Wt at 14 weeks: 27.5±0.34 g, n=15; P<0.05); however, from 17 weeks onward, body weight did not differ between Tg and Wt mice (Figure 2a). In contrast, BMP-3b Tg mice fed HFD weighed significantly less than did HFD-fed Wt mice throughout the observation period (Tg at 29 weeks: 49.1±1.1 g, n=15; Wt at 29 weeks: 57.9±1.5 g, n=14; P<0.01; Figure 2a). In addition, the decreased weight gain of BMP-3b Tg mice on HFD was overtly apparent (Figure 2b). These data indicate that BMP-3b Tg mice exhibited reduced adiposity on both diets, but because the effects were greater in mice on HFD than STD, we focused on the effects of BMP-3b in the context of HFD feeding in subsequent experiments.
BMP-3b Tg mice are protected against diet-induced obesity. Wt and Tg mice were fed STD or a HFD. (a) Body weights of Wt and Tg mice on STD and HFD. (STD: Wt, n=7–23; Tg, n=5–22; HFD: Wt, n=8–19; Tg, n=11–21; the number of mice used every week is shown in Supplementary Table 2). *P<0.05; **P<0.01 compared with Wt. (b) Macroscopic appearance of 30-week-old Wt and Tg mice fed HFD since the age of 5 weeks. (c) Weights of the peri, subQ, mes and epi fat pads, and of BAT in Wt and Tg mice on HFD (age, 30 weeks; Wt, n=6; Tg, n=4–9). *P<0.05; **P<0.01 compared with Wt. (d) Macroscopic appearance of perirenal fat pads from Wt and Tg mice on HFD (c). Scale bars, 5 mm. (e) Hematoxylin and eosin staining and average adipocyte area (n=4), and distribution by area of adipocytes within the subcutaneous fat pad in mice on HFD (scale bars, 200 μm). **P<0.01 compared with Wt. BAT, brown adipose tissue; epi, epididymal fat pads; mes, mesenteric; peri, perirenal; subQ, subcutaneous.
Fat-pad weights were decreased in BMP-3b Tg compared with Wt mice (Figure 2c). In particular, the perirenal fat pad weighed significantly less in BMP-3b Tg mice fed HFD than in corresponding Wt control mice (48% reduction; Tg, n=8; Wt, n=12; P<0.01; Figures 2c and d). Histological analysis revealed that adipocyte area was smaller in BMP-3b Tg mice than in Wt mice (Figure 2e).
The expression of both PPARγ and fatty acid translocase was downregulated in the adipose tissue of BMP-3b Tg mice on HFD
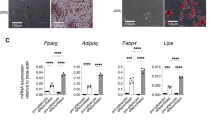
Because exogenous BMP-3b suppressed the adipogenesis of cultured adipocytes in our previous study,16 we analyzed the gene expression of key markers of adipogenesis in white adipose tissues and found decreased expression of PPARγ and C/EBPα, key regulators of adipogenesis, in BMP-3b Tg mice compared with Wt controls (Figure 3a); these findings are consistent with the effects of BMP-3b on adipogenesis in vitro, such as in 3T3-L1 cells and primary cultured adipocytes from adipose tissues.16 In contrast, the expression levels of C/EBPβ, C/EBPδ, sterol regulatory element binding protein 1c (SREBP-1c) and preadipocyte factor 1 (Pref-1) did not differ significantly between BMP-3b Tg mice and Wt mice. We then examined three markers of lipogenesis in white adipose tissues (Figure 3b) and found that the expression of fatty acid translocase (FAT) (also called CD36), a known PPARγ target gene that is involved in fatty acid uptake,21, 22, 23 was decreased in BMP-3b Tg mice compared with Wt controls. Furthermore, BMP-3b Tg mice had fewer mRNA transcripts of perilipin, which affects lipid droplet synthesis in adipocytes.
The expression of PPARγ and FAT in visceral fat is suppressed in BMP-3b Tg mice on HFD. (a, b) Expression of genes associated with adipogenesis (a) and lipogenesis (b) in perirenal fat of Wt and BMP-3b Tg mice on HFD (age, 30 weeks; n=5 or 6). Expression levels were normalized according to S18 mRNA levels, as described in the Materials and Methods section. Normalized values from Wt mice were arbitrarily assigned a value of 1. *P<0.05; **P<0.01 compared with Wt.
BMP-3b Tg mice on HFD showed improved glucose tolerance and increased insulin sensitivity
To reveal the systemic manifestations of the reduced adiposity in BMP-3b Tg mice, we performed glucose and insulin tolerance tests. Blood glucose levels after the administration of glucose or insulin were significantly lower in BMP-3b Tg mice than in Wt mice (Figure 4a). In addition, the leptin level was decreased and the adiponectin concentration was increased in the serum of BMP-3b Tg mice (Figure 4b). These data reflect the observed reductions in adipose tissue mass (Figures 2c and d) and adipocyte area (Figure 2e). Moreover, the serum total cholesterol level, which is usually elevated in HFD-induced obese mice, was decreased in BMP-3b Tg mice compared with Wt mice (Figure 4b). However, serum levels of insulin, triglycerides and nonesterified fatty acids did not differ significantly between Tg and Wt mice.
BMP-3b Tg mice on HFD show improved glucose tolerance and insulin sensitivity. (a) Results of glucose tolerance tests (GTTs) and insulin tolerance tests (ITTs) in Wt and BMP-3b Tg mice. Blood glucose was measured at 0, 15, 30, 60 and 120 min after the injection of glucose or insulin in Wt (open symbols) and BMP-3b Tg (filled symbols) mice (n=5 per group). *P<0.05; **P<0.01 compared with Wt. (b) Serum leptin, insulin, adiponectin, total cholesterol, triglyceride and nonesterified fatty acid (NEFA) levels in Wt and Tg mice on HFD (n=5 per group). *P<0.05; **P<0.01 compared with Wt.
BMP-3b Tg mice on HFD showed enhanced energy expenditure
To determine the mechanism underlying the protective effect of BMP-3b against increases in body and fat-pad weights, and adipocyte hypertrophy in BMP-3b Tg mice on HFD, we compared the food intake, locomotor activity and oxygen consumption of BMP-3b Tg mice with those of Wt mice (Figure 5). Despite their decreased weight gain, BMP-3b Tg mice showed significantly increased intake of HFD during the dark phase (Figure 5a). Ambulatory activity was higher in BMP-3b Tg mice than in Wt mice during the dark phase (Figure 5b), and, regardless of photoperiod, oxygen consumption was greater in BMP-3b Tg mice than in Wt mice, generating an overall increase in energy expenditure (Figure 5c). However, the respiratory quotient did not differ between BMP-3b Tg and Wt mice (Figure 5c). Overall, our results indicate that BMP-3b overexpression prevents HFD-induced obesity and glucose intolerance, and increases energy expenditure in mice.
BMP-3b Tg mice on HFD show enhanced energy expenditure. (a) Food intake, (b) locomotor activity and (c) oxygen consumption (VO2) during the light and dark phases of the photoperiod, average VO2, energy expenditure and respiratory quotient of Wt and Tg mice (age, 11 weeks; n=5) were determined by indirect calorimetry. *P<0.05; **P<0.01 compared with Wt.
Discussion
BMP-3b was initially isolated from rat and human femur,10, 11 and acts as a negative regulator of osteogenesis through Smad2/3 signaling.13, 15 To identify the role of BMP-3b in adult tissues other than bone, we focused on adipose tissues, in which the expression of BMP-3b was nearly equal to that in bone.16 In addition, both osteoblasts and adipocytes are differentiated from mesenchymal stem cells,24 further supporting the notion that BMP-3b might modulate adipocyte function. In fact, in our previous study, BMP-3b and its receptors (ALK-4 and ActRIIA) were expressed in mouse adipocytes, and BMP-3b inhibited the adipogenesis of several types of preadipocytes, including 3T3-L1 cells and primary cultures of mouse preadipocytes, by suppressing PPARγ.16 Moreover, we found that exogenous BMP-3b inhibited the adipogenesis of human cultured preadipocytes by suppressing PPARγ (data not shown), and the expression of human BMP-3b reportedly is elevated in the adipose tissue of overweight women.17
In the present study, we investigated the function of BMP-3b by using Tg mice that overexpressed BMP-3b in adipose tissue. Compared with Wt controls, HFD-fed BMP-3b Tg mice showed reductions in body weight, fat-pad weight and adipocyte area, indicating that BMP-3b prevents HFD-induced obesity. Because BMP-3b inhibits adipogenesis in cultured preadipocytes,16 the effects noted in BMP-3b Tg mice might similarly be due to the suppression of adipogenesis. We found that the gene expression levels of PPARγ and C/EBPα, key regulators of adipogenesis were suppressed in the white adipose tissues of BMP-3b Tg mice on HFD (Figure 3); these results are in good agreement with the described effects of BMP-3b on cultured preadipocytes in vitro.16 In terms of intracellular signaling, Smad2 phosphorylation was increased in the adipose tissue of BMP-3b Tg mice (Figure 1d); consistent with this finding is the report that phosphorylated Smad2 suppresses the expression of PPARγ and C/EBPα, and thus their effects on adipocytes.25 In addition, heterozygous PPARγ-deficient (PPARγ+/−) mice showed protection against HFD-induced body weight gain, increases in adipose tissue weight and adipocyte hypertrophy;22, 26 these phenotypes are similar to those of BMP-3b Tg mice on HFD. Notably, as in PPARγ+/− mice, the expression of FAT,21, 22, 23 a gene target of PPARγ that is involved in fatty acid uptake, was downregulated in the white adipose tissues of HFD-fed BMP-3b Tg mice (Figure 3). In addition, fatty acid uptake was reduced in adipocytes of FAT-null mice.20 These data suggest that overexpression of BMP-3b inhibited the translocation of fatty acids into adipocytes by suppressing PPARγ, thereby reducing adipocyte area and fat-pad weight; these phenotypes might be due in part to the downregulation of FAT and perilipin, which are involved in lipogenesis. We also examined the effects of BMP-3b Tg mice on STD in terms of fat-pad weight, adipocyte area and glucose metabolism. We found that STD either had no effect on BMP-3b Tg mice or that its effect was similar to that in HFD-fed BMP-3b Tg mice (data not shown). We are now exploring the precise mechanism by which BMP-3b regulates adipocyte functions in BMP-3b Tg mice on both HFD and STD.
Given that glucose intolerance and insulin insensitivity are commonly associated with increased body weight,27 the improved glucose tolerance and increased insulin sensitivity in BMP-3b Tg mice on HFD (Figure 4a) likely result from the reduction of body weight gain in these mice. However, an alternative mechanism, such as direct or indirect effects of BMP-3b on glucose metabolism or insulin signaling, also is plausible. For example, the expression of BMP-3b was increased in liver, where glucose metabolism is regulated by insulin signaling, in BMP-3b Tg mice compared with Wt mice both on STD and HFD (Figure 1 and Supplementary Figure 1). We currently are further investigating the hepatic phenotype of BMP-3b Tg mice on HFD.
Although the increase in body weight (characteristic of HFD-induced obesity) was inhibited in BMP-3b Tg mice, their food intake during the dark phase was significantly greater than that of Wt mice. These results and the increased metabolic rate of BMP-3b Tg mice suggest that the energy expenditure that they achieved through their increased locomotor activity and oxygen consumption exceeded the energy they acquired through increased food intake.
Compared with Wt controls, BMP-3b Tg mice on HFD displayed increased locomotor activity, which likely be caused by their reduced body weight gain and decreased fat-pad weights. In addition, we found that the expression levels of UCP1 and Cidea, which are key modulators of brown adipocyte function, were upregulated in the brown adipose tissue of HFD-fed BMP-3b Tg mice (Supplementary Figure 3a); this effect contributes to the increased energy expenditure in these animals. We also examined the expression of UCP1 in the subcutaneous fat tissue, where white fat cells can become beige fat cells, and found that it did not differ significantly between BMP-3b Tg and Wt mice (Supplementary Figure 3b). However, because energy expenditure is regulated in various tissues and through various systems, including the endocrine, neuroendocrine and nervous systems, additional studies are warranted to clarify the mechanism through which BMP-3b regulates the energy expenditure accompanying locomotor activity in BMP-3b Tg mice. In addition, we currently are using BMP-3b knockout mice to investigate the physiological significance of BMP-3b in energy expenditure.
Taken together, our data indicate that the overexpression of BMP-3b in adipose tissues renders mice resistant to diet-induced obesity and alters their metabolic homeostasis. These effects may be mediated, at least in part, through the suppression of PPARγ and FAT expression in adipose tissues. In summary, our study suggests that BMP-3b has beneficial effects against obesity and is a potential therapeutic target in counteracting obesity and related disorders.
References
Karastergiou K, Mohamed-Ali V . The autocrine and paracrine roles of adipokines. Mol Cell Endocrinol 2010; 318: 69–78.
White UA, Stephens JM . Transcriptional factors that promote formation of white adipose tissue. Mol Cell Endocrinol 2010; 318: 10–14.
Lefterova MI, Lazar MA . New developments in adipogenesis. Trends Endocrinol Metab 2009; 20: 107–114.
Rosen ED, Spiegelman BM . Adipocytes as regulators of energy balance and glucose homeostasis. Nature 2006; 444: 847–853.
Ahima RS, Lazar MA . Adipokines and the peripheral and neural control of energy balance. Mol Endocrinol 2008; 22: 1023–1031.
Rosen ED, MacDougald OA . Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol 2006; 7: 885–896.
Zamani N, Brown CW . Emerging roles for the transforming growth factor-beta superfamily in regulating adiposity and energy expenditure. Endocr Rev 2011; 32: 387–403.
Schulz TJ, Tseng YH . Emerging role of bone morphogenetic proteins in adipogenesis and energy metabolism. Cytokine Growth Factor Rev 2009; 20: 523–531.
Tseng YH, Kokkotou E, Schulz TJ, Huang TL, Winnay JN, Taniguchi CM et al. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature 2008; 454: 1000–1004.
Takao M, Hino J, Takeshita N, Konno Y, Nishizawa T, Matsuo H et al. Identification of rat bone morphogenetic protein-3b (BMP-3b), a new member of BMP-3. Biochem Biophys Res Commun 1996; 219: 656–662.
Hino J, Takao M, Takeshita N, Konno Y, Nishizawa T, Matsuo H et al. cDNA cloning and genomic structure of human bone morphogenetic protein-3b (BMP-3b). Biochem Biophys Res Commun 1996; 223: 304–310.
Cunningham NS, Jenkins NA, Gilbert DJ, Copeland NG, Reddi AH, Lee SJ . Growth/differentiation factor-10: a new member of the transforming growth factor-beta superfamily related to bone morphogenetic protein-3. Growth Factors 1995; 12: 99–109.
Hino J, Kangawa K, Matsuo H, Nohno T, Nishimatsu S . Bone morphogenetic protein-3 family members and their biological functions. Front Biosci 2004; 9: 1520–1529.
Hino J, Nishimatsu S, Nagai T, Matsuo H, Kangawa K, Nohno T . Coordination of BMP-3b and Cerberus is required for head formation of Xenopus embryos. Dev Biol 2003; 260: 138–157.
Matsumoto Y, Otsuka F, Hino J, Miyoshi T, Takano M, Miyazato M et al. Bone morphogenetic protein-3b (BMP-3b) inhibits osteoblast differentiation via Smad2/3 pathway by counteracting Smad1/5/8 signaling. Mol Cell Endocrinol 2012; 350: 78–86.
Hino J, Miyazawa T, Miyazato M, Kangawa K . Bone morphogenetic protein-3b (BMP-3b) is expressed in adipocytes and inhibits adipogenesis as a unique complex. Int J Obes 2012; 36: 725–734.
Korsic M, Gotovac K, Nikolac M, Dusek T, Skegro M, Muck-Seler D et al. Gene expression in visceral and subcutaneous adipose tissue in overweight women. Front Biosci (Elite Ed) 2012; 4: 2834–2844.
Kawamoto S, Niwa H, Tashiro F, Sano S, Kondoh G, Takeda J et al. A novel reporter mouse strain that expresses enhanced green fluorescent protein upon Cre-mediated recombination. FEBS Lett 2000; 470: 263–268.
Nuotio-Antar AM, Poungvarin N, Li M, Schupp M, Mohammad M, Gerard S et al. FABP4-Cre mediated expression of constitutively active ChREBP protects against obesity, fatty liver, and insulin resistance. Endocrinology 2015; 156: 4020–4032.
Makowski L, Boord JB, Maeda K, Babaev VR, Uysal KT, Morgan MA et al. Lack of macrophage fatty-acid-binding protein aP2 protects mice deficient in apolipoprotein E against atherosclerosis. Nat Med 2001; 7: 699–705.
Teboul L, Febbraio M, Gaillard D, Amri EZ, Silverstein R, Grimaldi PA . Structural and functional characterization of the mouse fatty acid translocase promoter: activation during adipose differentiation. Biochem J 2001; 360: 305–312.
Rieusset J, Touri F, Michalik L, Escher P, Desvergne BA, Niesor E et al. A new selective peroxisome proliferator-activated receptor gamma antagonist with antiobesity antidiabetic activity. Mol Endocrinol 2002; 16: 2628–2644.
Coburn CT, Knapp FF, Febbraio M, Beets AL, Silverstein RL, Abumrad NA . Defective uptake and utilization of long chain fatty acids in muscle and adipose tissues of CD36 knockout mice. J Biol Chem 2000; 275: 32523–32529.
Baksh D, Song L, Tuan RS . Adult mesenchymal stem cells: characterization, differentiation, and application in cell and gene therapy. J Cell Mol Med 2004; 8: 301–316.
Yogosawa S, Izumi T . Roles of activin receptor-like kinase 7 signaling and its target, peroxisome proliferator-activated receptor gamma, in lean and obese adipocytes. Adipocyte 2013; 2: 246–250.
Kubota N, Terauchi Y, Miki H, Tamemoto H, Yamauchi T, Komeda K et al. PPAR gamma mediates high-fat diet-induced adipocyte hypertrophy and insulin resistance. Mol Cell 1999; 4: 597–609.
Olefsky J, Reaven GM, Farquhar JW . Effects of weight reduction on obesity - studies of lipid and carbohydrate metabolism in normal and hyperlipoproteinemic subjects. J Clin Invest 1974; 53: 64–76.
Acknowledgements
We thank Dr J Miyazaki for providing the pCAG-CAT-EGFP plasmid, Dr M Yoshimoto for guidance and helpful advice on indirect calorimetry, and Dr K Miura for helpful advice on western blotting. We appreciate the excellent technical assistance of Ms M Miyazaki, Ms Y Fujii and Ms M Kitazume. This work was supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan; the Intramural Research Fund (27-2-2) for Cardiovascular Diseases of the National Cerebral and Cardiovascular Center of Japan; the Japan Vascular Disease Research Foundation; and the Takeda Scientific Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on International Journal of Obesity website
Rights and permissions
About this article
Cite this article
Hino, J., Nakatani, M., Arai, Y. et al. Overexpression of bone morphogenetic protein-3b (BMP-3b) in adipose tissues protects against high-fat diet-induced obesity. Int J Obes 41, 483–488 (2017). https://doi.org/10.1038/ijo.2017.15
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ijo.2017.15
This article is cited by
-
Growth differentiation factor 10 inhibits fat infiltration in tongue muscles of mice with high-fat diet
Skeletal Muscle (2025)
-
Reduced plasma GDF10 levels are positively associated with cholesterol impairment and childhood obesity
Scientific Reports (2024)
-
Bmp8a deletion leads to obesity through regulation of lipid metabolism and adipocyte differentiation
Communications Biology (2023)
-
Endocrine role of bone in the regulation of energy metabolism
Bone Research (2021)