Abstract
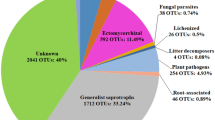
Coniferous forests cover extensive areas of the boreal and temperate zones. Owing to their primary production and C storage, they have an important role in the global carbon balance. Forest disturbances such as forest fires, windthrows or insect pest outbreaks have a substantial effect on the functioning of these ecosystems. Recent decades have seen an increase in the areas affected by disturbances in both North America and Europe, with indications that this increase is due to both local human activity and global climate change. Here we examine the structural and functional response of the litter and soil microbial community in a Picea abies forest to tree dieback following an invasion of the bark beetle Ips typographus, with a specific focus on the fungal community. The insect-induced disturbance rapidly and profoundly changed vegetation and nutrient availability by killing spruce trees so that the readily available root exudates were replaced by more recalcitrant, polymeric plant biomass components. Owing to the dramatic decrease in photosynthesis, the rate of decomposition processes in the ecosystem decreased as soon as the one-time litter input had been processed. The fungal community showed profound changes, including a decrease in biomass (2.5-fold in the litter and 12-fold in the soil) together with the disappearance of fungi symbiotic with tree roots and a relative increase in saprotrophic taxa. Within the latter group, successive changes reflected the changing availability of needle litter and woody debris. Bacterial biomass appeared to be either unaffected or increased after the disturbance, resulting in a substantial increase in the bacterial/fungal biomass ratio.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Adams HD, Macalady AK, Breshears DD, Allen CD, Stephenson NL, Saleska SR, Huxman TE, McDowell Ng . (2010). Climate-induced tree mortality: Earth system consequences. Eos, Trans Amer Geophys Union 91: 153–154.
Baldrian P, Kolařík M, Štursová M, Kopecký J, Valášková V, Větrovský T et al (2012). Active and total microbial communities in forest soil are largely different and highly stratified during decomposition. ISME J 6: 248–258.
Baldrian P, Větrovský T, Cajthaml T, Dobiášová P, Petránková M, Šnajdr J et al (2013). Estimation of fungal biomass in forest litter and soil. Fungal Ecol 6: 1–11.
Bače R, Svoboda M, Pouska V, Janda P, Červenka J . (2012). Natural regeneration in Central-European subalpine spruce forests: which logs are suitable for seedling recruitment? Forest Ecol Manag 266: 254–262.
Bouget C, Duelli P . (2004). The effects of windthrow on forest insect communities: a literature review. Biol Conserv 118: 281–299.
Bradshaw CJ, Warkentin IG, Sodhi NS . (2009). Urgent preservation of boreal carbon stocks and biodiversity. Trends Ecol Evol 24: 541–548.
Breshears DD, Myers OB, Meyer CW, Barnes FJ, Zou CB, Allen CD et al (2009). Tree die-off in response to global change-type drought: mortality insights from a decade of plant water potential measurements. Front Ecol Environ 7: 185–189.
Brunner I, Bakker MR, Bjork RG, Hirano Y, Lukac M, Aranda X et al (2013). Fine-root turnover rates of European forests revisited: an analysis of data from sequential coring and ingrowth cores. Plant Soil 362: 357–372.
Bárta J, Applová M, Vaněk D, Krištůfková M, Šantrůčková H . (2010). Effect of available P and phenolics on mineral N release in acidified spruce forest: connection with lignin-degrading enzymes and bacterial and fungal communities. Biogeochemistry 97: 71–87.
Bååth E, Anderson TH . (2003). Comparison of soil fungal/bacterial ratios in a pH gradient using physiological and PLFA-based techniques. Soil Biol Biochem 35: 955–963.
Bödeker ITM, Nygren CMR, Taylor AFS, Olson A, Lindahl BD . (2009). ClassII peroxidase-encoding genes are present in a phylogenetically wide range of ectomycorrhizal fungi. ISME J 3: 1387–1395.
Chapin FS III, Matson PA, Vitousek P . (2012) Principles of Terrestrial Ecosystem Ecology. Springer Verlag: New York.
Clemmensen KE, Bahr A, Ovaskainen O, Dahlberg A, Ekblad A, Wallander H et al (2013). Roots and associated fungi drive long-term carbon sequestration in boreal forest. Science 339: 1615–1618.
Edgar RC . (2010). Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26: 2460–2461.
Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R . (2011). UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 27: 2194–2200.
Ekberg A, Buchmann N, Gleixner G . (2007). Rhizospheric influence on soil respiration and decomposition in a temperate Norway spruce stand. Soil Biol Biochem 39: 2103–2110.
Ekblad A, Wallander H, Godbold DL, Cruz C, Johnson D, Baldrian P et al (2013). The production and turnover of extramatrical mycelium of ectomycorrhizal fungi in forest soils: role in carbon cycling. Plant Soil 366: 1–27.
Giesler R, Högberg MN, Strobel BW, Richter A, Nordgren A, Högberg P . (2007). Production of dissolved organic carbon and low-molecular weight organic acids in soil solution driven by recent tree photosynthate. Biogeochemistry 84: 1–12.
Hartmann M, Howes CG, VanInsberghe D, Yu H, Bachar D, Christen R et al (2012). Significant and persistent impact of timber harvesting on soil microbial communities in Northern coniferous forests. ISME J 6: 2199–2218.
Hicke JA, Allen CD, Desai AR, Dietze MC, Hall RJ, Ted Hogg EH et al (2012). Effects of biotic disturbances on forest carbon cycling in the United States and Canada. Global Change Biol 18: 7–34.
Högberg MN, Högberg P . (2002). Extramatrical ectomycorrhizal mycelium contributes one-third of microbial biomass and produces, together with associated roots, half the dissolved organic carbon in a forest soil. New Phytol 154: 791–795.
Högberg MN, Högberg P, Myrold DD . (2007). Is microbial community composition in boreal forest soils determined by pH, C-to-N ratio, the trees, or all three? Oecologia 150: 590–601.
Högberg P, Nordgren A, Buchmann N, Taylor AFS, Ekblad A, Högberg MN et al (2001). Large-scale forest girdling shows that current photosynthesis drives soil respiration. Nature 411: 789–792.
Jonášová M, Prach K . (2004). Central-European mountain spruce (Picea abies (L.) Karst.) forests: regeneration of tree species after a bark beetle outbreak. Ecol Eng 23: 15–27.
Jonášová M, Prach K . (2008). The influence of bark beetles outbreak vs. salvage logging on ground layer vegetation in Central European mountain spruce forests. Biol Conserv 141: 1525–1535.
Kaňa J, Tahovská K, Kopáček J . (2013). Response of soil chemistry to forest dieback after bark beetle infestation. Biogeochemistry 113: 369–383.
Kluber LA, Smith JE, Myrold DD . (2011). Distinctive fungal and bacterial communities are associated with mats formed by ectomycorrhizal fungi. Soil Biol Biochem 43: 1042–1050.
Kopáček J, Cudlín P, Svoboda M, Chmelíková E, Kaňa J, Picek T . (2010). Composition of Norway spruce litter and foliage in atmospherically acidified and nitrogen-saturated Bohemian Forest stands, Czech Republic. Boreal Environ Res 15: 413–426.
Kopáček J, Kaňa J, Šantrůčková H, Porcal P, Hejzlar J, Picek T et al (2002). Physical, chemical, and biochemical characteristics of soils in watersheds of the Bohemian Forest lakes: I. Plešné Lake. Silva Gabreta 8: 43–62.
Kurz WA, Dymond CC, Stinson G, Rampley GJ, Neilson ET, Carroll AL et al (2008). Mountain pine beetle and forest carbon feedback to climate change. Nature 452: 987–990.
Lundberg DS, Lebeis SL, Paredes SH, Yourstone S, Gehring J, Malfatti S et al (2012). Defining the core Arabidopsis thaliana root microbiome. Nature 488: 86–90.
Moore DJP, Trahan NA, Wilkes P, Quaife T, Stephens BB, Elder K et al (2013). Persistent reduced ecosystem respiration after insect disturbance in high elevation forests. Ecol Lett 16: 731–737.
Myneni RB, Dong J, Tucker CJ, Kaufmann RK, Kauppi PE, Liski J et al (2001). A large carbon sink in the woody biomass of Northern forests. Proc Natl Acad Sci USA 98: 14784–14789.
Okland B, Bjornstad ON . (2006). A resource-depletion model of forest insect outbreaks. Ecology 87: 283–290.
Reeder J, Knight R . (2010). Rapidly denoising pyrosequencing amplicon reads by exploiting rank-abundance distributions. Nat Methods 7: 668–669.
Reininger V, Grunig CR, Sieber TN . (2012). Host species and strain combination determine growth reduction of spruce and birch seedlings colonized by root-associated dark septate endophytes. Environ Microbiol 14: 1064–1076.
Ruckstuhl KE, Johnson EA, Miyanishi K . (2008). Introduction. The boreal forest and global change. Phil Trans Royal Soc London Ser B 363: 2245–2249.
Sagova-Mareckova M, Cermak L, Novotna J, Plhackova K, Forstova J, Kopecky J . (2008). Innovative methods for soil DNA purification tested in soils with widely differing characteristics. Appl Environ Microbiol 74: 2902–2907.
Schelhaas MJ, Nabuurs GJ, Schuck A . (2003). Natural disturbances in the European forests in the 19th and 20th centuries. Global Change Biol 9: 1620–1633.
Seidl R, Schelhaas MJ, Lexer MJ . (2011). Unraveling the drivers of intensifying forest disturbance regimes in Europe. Global Change Biol 17: 2842–2852.
Soja AJ, Tchebakova NM, French NHF, Flannigan MD, Shugart HH, Stocks BJ et al (2007). Climate-induced boreal forest change: predictions versus current observations. Glob Planet Change 56: 274–296.
Svoboda M, Janda P, Nagel TA, Fraver S, Rejzek J, Bace R . (2012). Disturbance history of an old-growth sub-alpine Picea abies stand in the Bohemian Forest, Czech Republic. J Veg Sci 23: 86–97.
Tedersoo L, Nilsson RH, Abarenkov K, Jairus T, Sadam A, Saar I et al (2010). 454 Pyrosequencing and Sanger sequencing of tropical mycorrhizal fungi provide similar results but reveal substantial methodological biases. New Phytol 188: 291–301.
Tejesvi MV, Sauvola T, Pirttila AM, Ruotsalainen AL . (2013). Neighboring Deschampsia flexuosa and Trientalis europaea harbor contrasting root fungal endophytic communities. Mycorrhiza 23: 1–10.
Tellenbach C, Grunig CR, Sieber TN . (2011). Negative effects on survival and performance of Norway spruce seedlings colonized by dark septate root endophytes are primarily isolate-dependent. Environ Microbiol 13: 2508–2517.
Vacek J, Krejčí F . (2009). Forest Ecosystems in the Bohemian Forest National Park (in Czech). Lesnická práce: Kostelec nad Černými Lesy, Czech Republic, pp 512.
Voříšková J, Brabcová V, Cajthaml T, Baldrian P . (2014). Seasonal dynamics of fungal communities in a temperate forest soil. New Phytol 201: 269–278.
Větrovský T, Baldrian P . (2013). Analysis of soil fungal communities by amplicon pyrosequencing: current approaches to data analysis and the introduction of the pipeline SEED. Biol Fertil Soils 49: 1027–1037.
Waldrop MP, Zak DR . (2006). Response of oxidative enzyme activities to nitrogen deposition affects soil concentrations of dissolved organic carbon. Ecosystems 9: 921–933.
Wallander H, Johansson U, Sterkenburg E, Brandström Durling M, Lindahl BD . (2010). Production of ectomycorrhizal mycelium peaks during canopy closure in Norway spruce forests. New Phytol 187: 1124–1134.
White TJ, Bruns T, Lee S, Taylor J . (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innins MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR Protocols. Academic Press: San Diego, CA, pp 315–322.
Yarwood SA, Myrold DD, Högberg MN . (2009). Termination of belowground C allocation by trees alters soil fungal and bacterial communities in a boreal forest. FEMS Microbiol Ecol 70: 151–162.
Zeller B, Liu J, Buchmann N, Richter A . (2008). Tree girdling increases soil N mineralisation in two spruce stands. Soil Biol Biochem 40: 1155–1166.
Šantrůčková H, Krištůfková M, Vaněk D . (2006). Decomposition rate and nutrient release from plant litter of Norway spruce forest in the Bohemian Forest. Biologia 61: S499–S508.
Šnajdr J, Cajthaml T, Valášková V, Merhautová V, Petránková M, Spetz P et al (2011a). Transformation of Quercus petraea litter: successive changes in litter chemistry are reflected in differential enzyme activity and changes in the microbial community composition. FEMS Microbiol Ecol 75: 291–303.
Šnajdr J, Dobiášová P, Větrovský T, Valášková V, Alawi A, Boddy L et al (2011b). Saprotrophic basidiomycete mycelia and their interspecific interactions affect the spatial distribution of extracellular enzymes in soil. FEMS Microbiol Ecol 78: 80–90.
Šnajdr J, Valášková V, Merhautová V, Herinková J, Cajthaml T, Baldrian P . (2008). Spatial variability of enzyme activities and microbial biomass in the upper layers of Quercus petraea forest soil. Soil Biol Biochem 40: 2068–2075.
Štursová M, Baldrian P . (2011). Effects of soil properties and management on the activity of soil organic matter transforming enzymes and the quantification of soil-bound and free activity. Plant Soil 338: 99–110.
Štursová M, Žifčáková L, Leigh MB, Burgess R, Baldrian P . (2012). Cellulose utilization in forest litter and soil: identification of bacterial and fungal decomposers. FEMS Microbiol Ecol 80: 735–746.
Žifčáková L, Dobiášová P, Kolářová Z, Koukol O, Baldrian P . (2011). Enzyme activities of fungi associated with Picea abies needles. Fungal Ecol 4: 427–436.
Acknowledgements
This work was supported by the Ministry of Education, Youth and Sports of the Czech Republic (LD12050), by the Czech Science Foundation (526/08/0751, P504/12/0709) and by the research concept of the Institute of Microbiology of the ASCR (RVO61388971).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on The ISME Journal website
Rights and permissions
About this article
Cite this article
Štursová, M., Šnajdr, J., Cajthaml, T. et al. When the forest dies: the response of forest soil fungi to a bark beetle-induced tree dieback. ISME J 8, 1920–1931 (2014). https://doi.org/10.1038/ismej.2014.37
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ismej.2014.37
Keywords
This article is cited by
-
Large-diameter trees and deadwood correspond with belowground ectomycorrhizal fungal richness
Ecological Processes (2023)
-
Distribution and vegetation of Pinus mugo subsp. mugo dieback patches in Maiella massif (Central Italy)
European Journal of Forest Research (2022)
-
The effect of tree mortality on CO2 fluxes in an old-growth spruce forest
European Journal of Forest Research (2021)
-
The effects of ectomycorrhizal fungal networks on seedling establishment are contingent on species and severity of overstorey mortality
Mycorrhiza (2020)
-
Pathogen-induced tree mortality interacts with predicted climate change to alter soil respiration and nutrient availability in Mediterranean systems
Biogeochemistry (2019)