Abstract
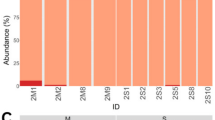
Ticks are of medical importance owing to their ability to transmit pathogens to humans and animals. The Rocky Mountain wood tick, Dermacentor andersoni, is a vector of a number of pathogens, including Anaplasma marginale, which is the most widespread tick-borne pathogen of livestock. Although ticks host pathogenic bacteria, they also harbor bacterial endosymbionts that have a role in tick physiology, survival, as well as pathogen acquisition and transmission. The goal of this study was to characterize the bacterial microbiome and examine the impact of microbiome disruption on pathogen susceptibility. The bacterial microbiome of two populations of D. andersoni with historically different susceptibilities to A. marginale was characterized. In this study, the microbiome was disrupted and then ticks were exposed to A. marginale or Francisella novicida to determine whether the microbiome correlated with pathogen susceptibility. Our study showed that an increase in proportion and quantity of Rickettsia bellii in the microbiome was negatively correlated to A. marginale levels in ticks. Furthermore, a decrease in Francisella endosymbionts was associated with lower F. novicida infection levels, demonstrating a positive pathogen–endosymbiont relationship. We demonstrate that endosymbionts and pathogens have varying interactions, and suggest that microbiome manipulation may provide a possible method for biocontrol by decreasing pathogen susceptibility of ticks.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Ahantarig A, Trinachartvanit W, Baimai V, Grubhoffer L . (2013). Hard ticks and their bacterial endosymbionts (or would be pathogens). Folia Microbiol (Praha) 58: 419–428.
Andreotti R, de León AAP, Dowd SE, Guerrero FD, Bendele KG, Scoles GA . (2011). Assessment of bacterial diversity in the cattle tick Rhipicephalus Boophilus microplus through tag-encoded pyrosequencing. BMC Microbiol 11: 6–6.
Bonnet S, Michelet L, Moutailler S, Cheval J, Hébert C, Vayssier-Taussat M et al. (2014). Identification of parasitic communities within european ticks using next-generation sequencing. PLoS Negl Trop Dis 8: e2753.
Brayton KA, Kappmeyer LS, Herndon DR, Dark MJ, Tibbals DL, Palmer GH et al. (2005). Complete genome sequencing of Anaplasma marginale reveals that the surface is skewed to two superfamilies of outer membrane proteins. PNAS 102: 844–849.
Budachetri K, Browning RE, Adamson SW, Dowd SE, Chao C-C, Ching W-M et al. (2014). An insight into the microbiome of the Amblyomma maculatum (Acari: Ixodidae). J Med Entomol 51: 119–129.
Carpi G, Cagnacci F, Wittekindt NE, Zhao F, Qi J, Tomsho LP et al. (2011). Metagenomic profile of the bacterial communities associated with Ixodes ricinus ticks. PLoS One 6: e25604.
Clay K, Fuqua C . (2010) The Tick Microbiome: Diversity, Distribution and Influence of the Internal Microbial Community for a Blood-Feeding Disease Vector. Critical Needs and Gaps in Understand Prevention, Amelioration, and Resolution of Lyme and Other Tick-Borne Diseases: the Short-Term and Long-Term Outcomes. Institute of Medicine Committee on Lyme Disease and Other Tick-Borne Diseases: The State of the Science: Washington DC, USA. Available from: http://www.iom.edu/~/media/Files/Activity%20Files/Disease/TickBorne/08-The-Tick-Microbiome.pdf.
Clay K, Klyachko O, Grindle N, Civitello D, Oleske D, Fuqua C . (2008). Microbial communities and interactions in the lone star tick Amblyomma americanum. Mol Ecol 17: 4371–4381.
Cole JR, Wang Q, Cardenas E, Fish J, Chai B, Farris RJ et al. (2009). The ribosomal database project: improved alignments and new tools for rRNA analysis. Nucleic Acids Res 37: D141–D145.
Cooley RA . (1938) The genera Dermacentor and Otocentor (Ixodidae) in the United States, with studies in variation. US Government Printing Office: Washington DC, USA.
Dumler JS, Barbet AF, Bekker CPJ, Dasch GA, Palmer GH, Ray SC et al. (2001). Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: unification of some species of Ehrlichia with Anaplasma Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and 'HGE agent' as subjective synonyms of Ehrlichia phagocytophila. Int J Syst Evol Microbiol 51: 2145–2165.
Eriks IS, Stiller D, Palmer GH . (1993). Impact of persistent Anaplasma marginale rickettsemia on tick infection and transmission. J Clin Microbiol 31: 2091–2096.
Futse JE, Ueti MW, Knowles DP Jr, Palmer GH . (2003). Transmission of Anaplasma marginale by Boophilus microplus: retention of vector competence in the absence of vector-pathogen interaction. J Clin Microbiol 41 (8): 3829–3834.
Graham RI, Grzywacz D, Mushobozi WL, Wilson K . (2012). Wolbachia in a major African crop pest increases susceptibility to viral disease rather than protests. Ecol Letter 15: 993–1000.
Halos L, Bord S, Cotte V, Gasqui P, Abrial D, Barnouin J et al. (2010). Ecological factors characterizing the prevalence of bacterial tick-borne pathogens in Ixodes ricinus ticks in pastures and woodlands. Appl Environ Microbiol 76: 4413–4420.
Hawlena H, Rynkiewicz E, Toh E, Alfred A, Durden LA, Hastriter MW et al. (2012). The arthropod, but not the vertebrate host or its environment, dictates bacterial community composition of fleas and ticks. ISME J 7: 221–223.
Herren JK, Lemaitre B . (2011). Spiroplasma and host immunity: activation of humoral immune responses increases endosymbiont load and susceptibility to certain Gram-negative bacterial pathogens in Drosophila melanogaster. Cell Microbiol 13: 1385–1396.
Jones RT, Knight R, Martin AP . (2009). Bacterial communities of disease vectors sampled across time, space, and species. ISME J 4: 223–231.
Jongejan F, Uilenberg G . (2004). The global importance of ticks. Int J Parasitol 129: S3.
Kocan KM, la Fuente De J, Guglielmone AA, Meléndez RD . (2003). Antigens and alternatives for control of Anaplasma marginale infection in cattle. Clin Microbiol Rev 16: 698–712.
Kuriwada T, Hosokawa T, Kumano N, Shiromoto K, Haraguchi D et al. (2010). Biological role of Nardonella Endosymbiont in its Weevil Host. PLoS One 5: e13101.
Lane DJ . (1991) 16S/23S rRNA sequencing. In: Stackebrandt E, Goodfellow M (eds), Nucleic acid techniques in bacterial systematics. John Wiley and Sons: New York, NY, USA, pp 115–175.
Macaluso KR, Sonenshine DE, Ceraul SM, Azad AF . (2002). Rickettsial infection in Dermacentor variabilis (Acari: Ixodidae) inhibits transovarial transmission of a second rickettsia. J Med Entomol 39: 809–813.
Niebylski ML, Peacock MG, Fischer ER, Porcella SF, Schwan TG . (1997). Characterization of an endosymbiont infecting wood ticks, Dermacentor andersoni, as a member of the genus Francisella. Appl Environ Microbiol 63: 3933–3940.
Oliver JD, Burkhardt NY, Felsheim RF, Kurtti TJ, Munderloh UG . (2014). Motility characteristics are altered for Rickettsia bellii transformed to overexpress a heterologous rickA gene. Appl Environ Microbiol 80: 1170–1176.
Reif KE, Palmer GH, Crowder DW, Ueti MW, Noh SM . (2014). Restriction of Francisella novicida genetic diversity during infection of the vector midgut. PLoS Pathog 10: e1004499.
Reif KE, Palmer GH, Ueti MW, Scoles GA, Margolis JJ, Monack DM et al. (2011). Dermacentor andersoni transmission of Francisella tularensis subsp. novicida reflects bacterial colonization, dissemination, and replication coordinated with tick feeding. Infect Immun 79: 4941–4946.
Rochon K, Scoles GA, Lysyk TJ . (2012). Dispersion and sampling of Adult Dermacentor andersoni in rangeland in Western North America. J Med Entomol 49: 253–261.
Scoles GA, Ueti MW, Palmer GH . (2005). Variation among geographically separated populations of Dermacentor andersoni (Acari: Ixodidae) in midgut susceptibility to Anaplasma marginale (Rickettsiales: Anaplasmataceae). J Med Entomol 42: 153–162.
Telford SR III . (2009). Status of the ‘East Side Hypothesis’ (Transovarial Interference) 25 Years Later. Ann N Y Acad Sci 1166: 144–150.
Turner SW, Pryer KM, Miao VPW, Palmer JD . (1999). Investigating deep phylogenetic relationships among cyanobacteria and plastids by small subunit rRND sequence analysis. J Eukaryot Microbiol 46: 327–338.
Ueti MW, Reagan JO, Knowles DP, Scoles GA, Shkap V, Palmer GH . (2007). Identification of midgut and salivary glands as specific and distinct barriers to efficient tick-borne transmission of Anaplasma marginale. Infect Immun 75: 2959–2964.
Weiss B, Aksoy S . (2011). Microbiome influence on insect host vector competence. Trends Parasitol 27: 514–522.
Ze'le F, Nicot A, Berthomieu A, Weill M, Duron O, Rivero A . (2014). Wolbachia increases susceptibility to Plasmodium infection in a natural system. Proc R Soc B 281: 20132837–20132837.
Zhong J, Jasinskas A, Barbour AG . (2007). Antibiotic treatment of the tick vector Amblyomma americanum reduced reproductive fitness. PLoS One 2: e405.
Acknowledgements
We thank Lisa Orfe, Mark Wildung, Derek Pouchnik, Ralph Horn, James Allison and the staff at the USDA-ARS in Moscow, ID, USA for excellent guidance and technical assistance. This research was supported by National Institutes of Health grants, AI044005, AI093524, 5T32GM008336-25, www.experiment.com (crowdsourcing grant), USDA-ARS-CRIS 5348-32000-033-00D and CVM intramural funds.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on The ISME Journal website
Supplementary information
Rights and permissions
About this article
Cite this article
Gall, C., Reif, K., Scoles, G. et al. The bacterial microbiome of Dermacentor andersoni ticks influences pathogen susceptibility. ISME J 10, 1846–1855 (2016). https://doi.org/10.1038/ismej.2015.266
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ismej.2015.266
This article is cited by
-
Entomopathogenic fungus treatment changes the gut bacterial diversity of Rhipicephalus microplus ticks
Parasites & Vectors (2023)
-
New insights into the impact of microbiome on horizontal and vertical transmission of a tick-borne pathogen
Microbiome (2023)
-
Tick microbial associations at the crossroad of horizontal and vertical transmission pathways
Parasites & Vectors (2022)
-
Quantitative microbial population study reveals geographical differences in bacterial symbionts of Ixodes ricinus
Microbiome (2022)
-
Microbial composition in Hyalomma anatolicum collected from livestock in the United Arab Emirates using next-generation sequencing
Parasites & Vectors (2022)