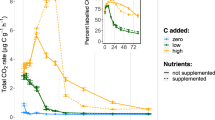
Abstract
CO2 release from soil is commonly used to estimate toxicity of various substances on microorganisms. However, the mechanisms underlying persistent CO2 release from soil exposed to toxicants inhibiting microbial respiration, for example, sodium azide (NaN3) or heavy metals (Cd, Hg, Cu), remain unclear. To unravel these mechanisms, NaN3-amended soil was incubated with position-specifically 13C-labeled glucose and 13C was quantified in CO2, bulk soil, microbial biomass and phospholipid fatty acids (PLFAs). High 13C recovery from C-1 in CO2 indicates that glucose was predominantly metabolized via the pentose phosphate pathway irrespective of inhibition. Although NaN3 prevented 13C incorporation into PLFA and decreased total CO2 release, 13C in CO2 increased by 12% compared with control soils due to an increased use of glucose for energy production. The allocation of glucose-derived carbon towards extracellular compounds, demonstrated by a fivefold higher 13C recovery in bulk soil than in microbial biomass, suggests the synthesis of redox active substances for extracellular disposal of electrons to bypass inhibited electron transport chains within the cells. PLFA content doubled within 10 days of inhibition, demonstrating recovery of the microbial community. This growth was largely based on recycling of cost-intensive biomass compounds, for example, alkyl chains, from microbial necromass. The bypass of intracellular toxicity by extracellular electron transport permits the fast recovery of the microbial community. Such efficient strategies to overcome exposure to respiration-inhibiting toxicants may be exclusive to habitats containing redox-sensitive substances. Therefore, the toxic effects of respiration inhibitors on microorganisms are much less intensive in soils than in pure cultures.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Apostel C, Dippold M, Glaser B, Kuzyakov Y . (2013). Biochemical pathways of amino acids in soil: assessment by position-specific labeling and 13C-PLFA analysis. Soil Biol Biochem 67: 31–40.
Apostel C, Dippold M, Kuzyakov Y . (2015). Biochemistry of hexose and pentose transformations in soil analyzed by position-specific labeling and 13C-PLFA. Soil Biol Biochem 80: 199–208.
Ausmus BS, Dodson GJ, Jackson DR . (1978). Behavior of heavy-metal in forest microcosms. 3. Effects on litter-carbon metabolism. Water Air Soil Pollut 10: 19–26.
Babich H, Stotzky G . (1985). Heavy metal toxicity to microbe-mediated ecologic processes: a review and potential application to regulatory policies. Environ Res 36: 111–137.
Belyaeva EA, Sokolova TV, Emelyanova LV, Zakharova IO . (2012). Mitochondrial electron transport chain in heavy metal-induced neurotoxicity: effects of cadmium, mercury, and copper. Sci World J 2012: 136063–136063.
Bi R, Lu Q, Yu W, Yuan Y, Zhou S . (2013). Electron transfer capacity of soil dissolved organic matter and its potential impact on soil respiration. J Soil Sediment 13: 1553–1560.
Blagodatskaya E, Kuzyakov Y . (2013). Active microorganisms in soil: critical review of estimation criteria and approaches. Soil Biol Biochem 67: 192–211.
Blagodatskaya E, Yuyukina T, Blagodatsky S, Kuzyakov Y . (2011). Three-source-partitioning of microbial biomass and of CO2 efflux from soil to evaluate mechanisms of priming effects. Soil Biol Biochem 43: 778–786.
Blankinship JC, Becerra CA, Schaeffer SM, Schimel JP . (2014). Separating cellular metabolism from exoenzyme activity in soil organic matter decomposition. Soil Biol Biochem 71: 68–75.
Bond H, Lighthart B, Shimabuku R, Russell L . (1976). Some effects of cadmium on coniferous forest soil and litter microcosms. Soil Sci 121: 278–287.
Bremer E, van Kessel C . (1990). Extractability of microbial 14C and 15N following addition of variable rates of labelled glucose and (NH4 2SO4 to soil. Soil Biol Biochem 22: 707–713.
Caspi R, Foerster H, Fulcher CA, Kaipa P, Krummenacker M, Latendresse M et al. (2008). The MetaCyc database of metabolic pathways and enzymes and the BioCyc collection of Pathway/Genome Databases. Nucleic Acids Res 36: D623–D631.
Dijkstra P, Dalder JJ, Selmants PC, Hart SC, Koch GW, Schwartz E et al. (2011a). Modeling soil metabolic processes using isotopologue pairs of position-specific C-13-labeled glucose and pyruvate. Soil Biol Biochem 43: 1848–1857.
Dijkstra P, Salpas E, Fairbanks D, Miller EB, Hagerty SB, van Groenigen KJ et al. (2015). High carbon use efficiency in soil microbial communities is related to balanced growth, not storage compound synthesis. Soil Biol Biochem 89: 35–43.
Dijkstra P, Thomas SC, Heinrich PL, Koch GW, Schwartz E, Hungate BA . (2011b). Effect of temperature on metabolic activity of intact microbial communities: evidence for altered metabolic pathway activity but not for increased maintenance respiration and reduced carbon use efficiency. Soil Biol Biochem 43: 2023–2031.
Dippold M, Biryukov M, Kuzyakov Y . (2014). Sorption affects amino acid pathways in soil: implications from position-specific labeling of alanine. Soil Biol Biochem 72: 180–192.
Dippold MA, Kuzyakov Y . (2013). Biogeochemical transformations of amino acids in soil assessed by position-specific labelling. Plant Soil 373: 385–401.
Dippold MA, Kuzyakov Y . (2016). Direct incorporation of fatty acids into microbial phospholipids in soils: position-specific labeling tells the story. Geochim Cosmochim Acta 174: 211–221.
EFSA. (2016). Recovery in environmental risk assessments at EFSA. EFSA J 14: 4313.
Fischer H, Ingwersen J, Kuzyakov Y . (2010). Microbial uptake of low-molecular-weight organic substances out-competes sorption in soil. Eur J Soil Sci 61: 504–513.
Fischer H, Meyer A, Fischer K, Kuzyakov Y . (2007). Carbohydrate and amino acid composition of dissolved organic matter leached from soil. Soil Biol Biochem 39: 2926–2935.
Fliessbach A, Martens R, Reber HH . (1994). Soil microbial biomass and microbial activity in soils treated with heavy metal contaminated sewage sludge. Soil Biol Biochem 26: 1201–1205.
Frostegård Å, Tunlid A, Bååth E . (1991). Microbial biomass measured as total lipid phosphate in soils of different organic content. J Microbiol Methods 14: 151–163.
Fuhrer T, Sauer U . (2009). Different biochemical mechanisms ensure network-wide balancing of reducing equivalents in microbial metabolism. J Bacteriol 191: 2112–2121.
Gearing JN . (1991). The study of diet and trophic relationships through natural abundance 13C. In: Coleman DC, Fry B (eds). Carbon Isotope Techniques. Academic Press: New York, pp 201–218..
Giller KE, Witter E, McGrath SP . (1998). Toxicity of heavy metals to microorganisms and microbial processes in agricultural soils: a review. Soil Biol Biochem 30: 1389–1414.
Glaser B, Amelung W . (2002). Determination of C-13 natural abundance of amino acid enantiomers in soil: methodological considerations and first results. Rapid Commun Mass Spectrom 16: 891–898.
Gorby YA, Yanina S, McLean JS, Rosso KM, Moyles D, Dohnalkova A et al. (2006). Electrically conductive bacterial nanowires produced by Shewanella oneidensis strain MR-1 and other microorganisms. Proc Natl Acad Sci USA 103: 11358–11363.
Gordon AS, Harwood VJ, Sayyar S . (1993). Growth, copper-tolerant cells, and extracellular protein production in copper-stressed chemostat cultures of Vibrio alginolyticus. Appl Environ Microbiol 59: 60–66.
Gunina A, Dippold MA, Glaser B, Kuzyakov Y . (2014). Fate of low molecular weight organic substances in an arable soil: from microbial uptake to utilisation and stabilisation. Soil Biol Biochem 77: 304–313.
Gunina A, Kuzyakov Y . (2015). Sugars in soil and sweets for microorganisms: review of origin, content, composition and fate. Soil Biol Biochem 90: 87–100.
ISO 16072. (2002) Soil quality—Laboratory Methods for Determination of Microbial Soil Respiration. International Organization for Standardization: Geneva, Switzerland.
Keilin D . (1936). The action of sodium azide on cellular respiration and on some catalytic oxidation reactions. Proc Roy Soc Lond B Biol Sci 121: 165–173.
Kelley WD, Rodriguez-Kabana R . (1981). Effects of annual applications of sodium azide on soil fungal populations with emphasis on Trichoderma species. Pestic Sci 12: 235–244.
Killham K . (1985). A physiological determination of the impact of environmental stress on microbial bomass. Environ Pollut A 38: 283–294.
Kramer C, Gleixner G . (2006). Variable use of plant- and soil-derived carbon by microorganisms in agricultural soils. Soil Biol Biochem 38: 3267–3278.
Kuzyakov Y . (2010). Priming effects: interactions between living and dead organic matter. Soil Biol Biochem 42: 1363–1371.
Lovley DR, Coates JD, BluntHarris EL, Phillips EJP, Woodward JC . (1996). Humic substances as electron acceptors for microbial respiration. Nature 382: 445–448.
Maire V, Alvarez G, Colombet J, Comby A, Despinasse R, Dubreucq E et al. (2013). An unknown oxidative metabolism substantially contributes to soil CO2 emissions. Biogeosciences 10: 1155–1167.
Mau RL, Liu CM, Aziz M, Schwartz E, Dijkstra P, Marks JC et al. (2015). Linking soil bacterial biodiversity and soil carbon stability. ISME J 9: 1477–1480.
McCarthy AJ, Williams ST . (1992). Actinomycetes as agents of biodegradation in the environment—a review. Gene 115: 189–192.
Monard C, Binet F, Vandenkoornhuyse P . (2008). Short-term response of soil bacteria to carbon enrichment in different soil microsites. Appl Environ Microbiol 74: 5589–5592.
Newman DK, Kolter R . (2000). A role for excreted quinones in extracellular electron transfer. Nature 405: 94–97.
Pentrakova L, Su K, Pentrak M, Stuck JW . (2013). A review of microbial redox interactions with structural Fe in clay minerals. Clay Miner 48: 543–560.
Piepenbrock A, Schroeder C, Kappler A . (2014). Electron transfer from humic substances to biogenic and abiogenic Fe(III) oxyhydroxide minerals. Environ Sci Technol 48: 1656–1664.
Reguera G, McCarthy KD, Mehta T, Nicoll JS, Tuominen MT, Lovley DR . (2005). Extracellular electron transfer via microbial nanowires. Nature 435: 1098–1101.
Roden EE, Kappler A, Bauer I, Jiang J, Paul A, Stoesser R et al. (2010). Extracellular electron transfer through microbial reduction of solid-phase humic substances. Nat Geosci 3: 417–421.
Rozycki M, Bartha R . (1981). Problems associated with the use of azide as an inhibitor of microbial activity in soil. Appl Environ Microbiol 41: 833–836.
Scandellari F, Hobbie EA, Ouimette AP, Stucker VK . (2009). Tracing metabolic pathways of lipid biosynthesis in ectomycorrhizal fungi from position-specific 13C-labelling in glucose. Environ Microbiol 11: 3087–3095.
Schimel J . (2013). Soil carbon microbes and global carbon. Nat Climate Change 3: 867–868.
Schmitt J, Glaser B, Zech W . (2003). Amount-dependent isotopic fractionation during compound-specific isotope analysis. Rapid Commun Mass Spectrom 17: 970–977.
Treonis AM, Ostle NJ, Stott AW, Primrose R, Grayston SJ, Ineson P . (2004). Identification of groups of metabolically-active rhizosphere microorganisms by stable isotope probing of PLFAs. Soil Biol Biochem 36: 533–537.
Trevors JT . (1996). Sterilization and inhibition of microbial activity in soil. J Microbiol Methods 26: 53–59.
Vempati RK, Kollipara KP, Stucki JW, Wilkinson H . (1995). Reduction of structural iron in selected iron-bearing minerals by soybean root exudates grown in an in-vitro geoponicsystem. J Plant Nutr 18: 343–353.
Voroney RP, Paul EA . (1984). Determination of KC and KN insitu for calibration of the chloroform fumigation incubation method. Soil Biol Biochem 16: 9–14.
Winter C, Kerros M-E, Weinbauer MG . (2012). Effects of sodium azide on the abundance of prokaryotes and viruses in marine samples. PLoS One 7: e37597.
Wittmann C, Weber J, Betiku E, Kroemer J, Boehm D, Rinas U . (2007). Response of fluxome and metabolome to temperature-induced recombinant protein synthesis in Escherichia coli. J Biotechnol 132: 375–384.
Wu J, Joergensen RG, Pommerening B, Chaussod R, Brookes PC . (1990). Measurement of soil microbial biomass C by fumigation extraction—an automated procedure. Soil Biol Biochem 22: 1167–1169.
Zelles L . (1999). Fatty acid patterns of phospholipids and lipopolysaccharides in the characterisation of microbial communities in soil: a review. Biol Fertil Soils 29: 111–129.
Zelles L, Bai QY, Rackwitz R, Chadwick D, Beese F . (1995). Determination of phospholipid-derived and lipopolysaccharide-derived fatty-acids as an estimate of microbial biomass and community structures in soils. Biol Fertil Soils 19: 115–123.
Acknowledgements
We thank the DFG for funding (DI-2136/1-1 and NTS 186/1006-1/P) and DAAD for funding Ezekiel Bore. We thank the technical staff of Goettingen University, in particular Karin Schmidt and Anita Kriegel, for microbial biomass C content determination, the entire team at KOSI (Centre for Stable Isotopes Analysis) for δ13C analysis and Joshua Bostic for English proofreading.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on The ISME Journal website
Supplementary information
Rights and permissions
About this article
Cite this article
K Bore, E., Apostel, C., Halicki, S. et al. Soil microorganisms can overcome respiration inhibition by coupling intra- and extracellular metabolism: 13C metabolic tracing reveals the mechanisms. ISME J 11, 1423–1433 (2017). https://doi.org/10.1038/ismej.2017.3
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ismej.2017.3
This article is cited by
-
Heavy metals might influence biological activities of soil close to the rhizosphere in a teak (Tectona grandis l.f.) plantation forest of India
Plant and Soil (2025)
-
Carbendazim Modulates the Metabolically Active Bacterial Populations in Soil and Rhizosphere
Current Microbiology (2023)
-
The presence of soybean, but not soybean cropping frequency has influence on SOM priming in crop rotation systems
Plant and Soil (2023)
-
Drought re-routes soil microbial carbon metabolism towards emission of volatile metabolites in an artificial tropical rainforest
Nature Microbiology (2023)
-
Heavy metal pollution increases soil microbial carbon limitation: Evidence from ecological enzyme stoichiometry
Soil Ecology Letters (2021)