Abstract
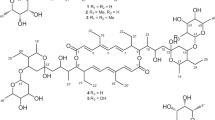
A new bafilomycin-type macrolide, named hygrobafilomycin (6), was isolated by a bioassay-guided selection and fractionation from a terrestrial actinomycete, Streptomyces varsoviensis, along with three known derivatives, bafilomycin D (3), C1 (4) and C2 (5). The structure of hygrobafilomycin was fully established by MS and NMR analyses, revealing a hygrolidin–bafilomycin hybrid with an unusual monoalkylmaleic anhydride moiety. Hygrobafilomycin (6) shows strong antifungal, antiproliferative and cytotoxic activities. In a panel of 40 tumor cell lines, compound 6 shows high cytotoxic potency (mean IC50=5.3 n).
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Seto, H., Tajima, I., Akao, H., Furihata, K. & Otake, N. The isolation and structures of hygrolidin amide and defumarylhygrolidin. J. Antibiot. 37, 610–613 (1984).
Goetz, M. A. et al. L-155,175—a new antiparasitic macrolide fermentation, isolation and structure. J. Antibiot. 38, 161–168 (1985).
Wilton, J. H., Hokanson, G. C. & French, J. C. Pd-118,576—a new antitumor macrolide antibiotic. J. Antibiot. 38, 1449–1452 (1985).
Kawada, M. et al. Hygrolidin induces p21 expression and abrogates cell cycle progression at G1 and S phases. Biochem. Biophys. Res. Commun. 298, 178–183 (2002).
Vanek, Z., Mateju, J. & Curdova, E. Immunomodulators isolated from microorganisms. Folia Microbiol. 36, 99–111 (1991).
Werner, G., Hagenmaier, H., Drautz, H., Baumgartner, A. & Zahner, H. Metabolic products of microorganisms 0.224. bafilomycins, a new group of macrolide antibiotics production, isolation, chemical-structure and biological-activity. J. Antibiot. 37, 110–117 (1984).
Kretschmer, A., Dorgerloh, M., Deeg, M. & Hagenmaier, H. The structures of novel insecticidal macrolides—bafilomycin-D and bafilomycin-E, and oxohygrolidin. Agric. Biol. Chem. 49, 2509–2511 (1985).
Kim, S.- D., Ryoo, I.- J., Kim, C.- J., Uramoto, M. & Yoo, I.- D. The structure determination of a herbicidal compound, 3D5. J. Microbiol. Biotech. 3, 51–56 (1993).
Drose, S. et al. Inhibitory effect of modified bafilomycins and concanamycins on P-type and V-type adenosine-triphosphatases. Biochemistry 32, 3902–3906 (1993).
Gagliardi, S., Rees, M. & Farina, C. Chemistry and structure activity relationships of bafilomycin A(1), a potent and selective inhibitor of the vacuolar H+-ATPase. Curr. Med. Chem. 6, 1197–1212 (1999).
Teplova, V. V., Tonshin, A. A., Grigoriev, P. A., Saris, N. E. L. & Salkinoja-Salonen, M. S. Bafilomycin A1 is a potassium ionophore that impairs mitochondrial functions. J. Bioenerg. Biomembr. 39, 321–329 (2007).
Bowman, E. J., Siebers, A. & Altendorf, K. Bafilomycins—a class of inhibitors of membrane Atpases from microorganisms, animal-cells, and plant-cells. Proc. Natl Acad. Sci. USA 85, 7972–7976 (1988).
Gagliardi, S. et al. Synthesis and structure-activity relationships of bafilomycin A(1) derivatives as inhibitors of vacuolar H+-ATPase. J. Med. Chem. 41, 1883–1893 (1998).
Bowman, E. J. & Bowman, B. J. V-ATPases as drug targets. J. Bioenerg. Biomembr. 37, 431–435 (2005).
Baker, G. H., Brown, P. J., Dorgan, R. J. J. & Everett, J. R. The conformational-analysis of bafilomycin-A1. J. Chem. Soc., Perkin Trans. 2, 1073–1079 (1989).
Seto, H., Akao, H., Furihata, K. & Otake, N. The structure of a new antibiotic, hygrolidin. Tetrahedron Lett. 23, 2667–2670 (1982).
Adlington, R. M., Baldwin, J. E., Cox, R. J. & Pritchard, G. J. A rapid entry to natural and unnatural disubstituted maleic anhydrides. Synlett. 820–822 (2002).
Kar, A. & Argade, N. P. A facile access to natural and unnatural dialkylsubstituted maleic anhydrides. Tetrahedron 59, 2991–2998 (2003).
Chen, X. L., Zheng, Y. G. & Shen, Y. C. Natural products with maleic anhydride structure: Nonadrides, tautomycin, chaetomellic anhydride, and other compounds. Chem. Rev. 107, 1777–1830 (2007).
Siddiqui, B. S., Afshan, F., Gulzar, T. & Hanif, M. Tetracyclic triterpenoids from the leaves of Azadirachta indica. Phytochemistry 65, 2363–2367 (2004).
Wang, N. et al. Sesterterpenoids from the sponge Sarcotragus sp. J. Nat. Prod. 71, 551–557 (2008).
Schuhmann, T. & Grond, S. Biosynthetic investigations of the v-type ATPase inhibitors bafilomycin A(1), B-1 and concanamycin A. J. Antibiot. 57, 655–661 (2004).
Schuhmann, T., Vollmar, D. & Grond, S. Biosynthetic origin of the methoxyl extender unit in bafilomycin and concanamycin using stereospecifically labeled precursors. J. Antibiot. 60, 52–60 (2007).
Hertweck, C. The biosynthetic logic of polyketide diversity. Angew. Chem. Int. Ed. 48, 4688–4716 (2009).
Li, W. L., Ju, J. H., Rajski, S. R., Osada, H. & Shen, B. Characterization of the tautomycin biosynthetic gene cluster from Streptomyces spiroverticillatus unveiling new insights into dialkylmaleic anhydride and polyketide biosynthesis. J. Biol. Chem. 283, 28607–28617 (2008).
Ubukata, M., Cheng, X. C., Uzawa, J. & Isono, K. Biosynthesis of the dialkylmaleic anhydride-containing antibiotics, tautomycin and tautomycetin. J. Chem. Soc., Perkin Trans. 1 2399–2404 (1995).
Ohta, T. et al. Bafilomycin A(1) induces apoptosis in the human pancreatic cancer cell line Capan-1. J. Pathol. 185, 324–330 (1998).
Fiebig, H. H. et al. High throughput screen for anticancer compounds based on human tumor cell lines and bioinformatics. Proc. Am. Assoc. Cancer Res. 46, 3967 (2005).
Dengler, W. A., Schulte, J., Berger, D. P., Mertelsmann, R. & Fiebig, H. H. Development of a propidium Iodide fluorescence assay for proliferation and cytotoxicity assays. Anti-Cancer Drugs 6, 522–532 (1995).
Acknowledgements
Financial support by the BMBF (FKZ 0315153) is gratefully acknowledged. We thank Heike Heinecke and Ulrike Valentin for technical assistance, Franziska Rhein for NMR measurements, Andrea Perner for MS measurements, Jutta Fehr and Roger Vollmer for cytotoxicity assays, Maria-Gabriele Schwinger and Christiane Weigel for antimicrobial assays.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on The Journal of Antibiotics website
Supplementary information
Rights and permissions
About this article
Cite this article
Tchize Ndejouong, B., Sattler, I., Maier, A. et al. Hygrobafilomycin, a cytotoxic and antifungal macrolide bearing a unique monoalkylmaleic anhydride moiety, from Streptomyces varsoviensis. J Antibiot 63, 359–363 (2010). https://doi.org/10.1038/ja.2010.52
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ja.2010.52
Keywords
This article is cited by
-
Biosynthesis of Methoxymalonyl-acyl Carrier Protein (ACP) as an Extender Unit for Bafilomycin Polyketide in Streptomyces griseus DSM 2608
Biotechnology and Bioprocess Engineering (2018)
-
Biosynthesis of 2-amino-3-hydroxycyclopent-2-enone moiety of bafilomycin in Kitasatospora cheerisanensis KCTC2395
Journal of Microbiology (2018)
-
Bafilomycin L, a new inhibitor of cholesteryl ester synthesis in mammalian cells, produced by marine-derived Streptomyces sp. OPMA00072
The Journal of Antibiotics (2015)
-
Antifungal Macrolides from Streptomyces cavourensis YY01-17
Chemistry of Natural Compounds (2013)
-
Antifungalmycin, an antifungal macrolide from Streptomyces padanus 702
Natural Products and Bioprospecting (2012)