Abstract
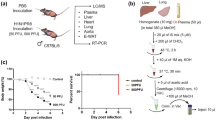
Severe respiratory disease arising from influenza virus infection has a high fatality rate. Neutrophil myeloperoxidase (MPO) has been implicated in the pathogenesis of severe influenza-induced pneumonia because extracellularly released MPO mediates the production of hypochlorous acid, a potent tissue injury factor. To search for candidate anti-influenza compounds, we screened leucomycin A3 (LM-A3), spiramycin (SPM), an erythromycin derivative (EM900, in which anti-bacterial activity has been eliminated), and clarithromycin (CAM), by analyzing their ability to inhibit MPO release in neutrophils from mice and humans. When each candidate was injected into mice infected with a lethal dose of A/H1N1 influenza virus (PR-8), LM-A3 produced the highest survival rate (80.9%). We found that LM-A3 induced beneficial effects on lung pathology and viral proliferation involved in the regulatory activity of MPO release, pro-inflammatory cytokines and interferon-α production in the lung. SPM and EM900 also induced positive survival effects in the infected mice, whereas CAM did not. We further found that these compounds inhibit virus proliferation in human pneumonia epithelial A549 cells in vitro. LM-A3 showed effective action against influenza A virus infection with high anti-viral activity in human host cells, indicating the possibility that LM-A3 is a prospective lead compound for the development of a drug for human influenza. The positive survival effect induced by EM900 suggests that pharmacological architectures between anti-bacterial and anti-influenza virus activities can be dissociated in macrolide derivatives. These observations provide valuable evidence for the potential development of novel macrolide derivatives that have strong anti-viral but no anti-bacterial activity.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Glezen, W. P., Taber, L. H., Frank, A. L., Gruber, W. C. & Piedra, P. A. Influenza virus infections in infants. Pediatr. Infect. Dis. J. 16, 1065–1068 (1997).
World Health Organization (WHO) Influenza (seasonal) fact sheet No. 211. Downloaded from http://www.who.int/mediacentre/factsheets/fs211/en/index.html (2009).
Luk, J., Gross, P. & Thompson, W. W. Observations on mortality during the 1918 influenza pandemic. Clin. Infect. Dis. 33, 1375–1378 (2001).
Tumpey, T. M. et al. Characterization of the reconstructed 1918 Spanish influenza pandemic virus. Science 310, 77–80 (2005).
Centers for Disease Control and Prevention (CDC). Cases of influenza A (H5N1)-Thailand, 2004. MMWR Morb. Mortal. Wkly Rep. 53, 100–103 (2004).
Tran, T. H. et al. Avian influenza A (H5N1) in 10 patients in Vietnam. N. Engl. J. Med. 350, 1179–1188 (2004).
Kobasa, D. et al. Aberrant innate immune response in lethal infection of macaques with the 1918 influenza virus. Nature 445, 319–323 (2007).
Kawachi, S. et al. Risk parameters of fulminant acute respiratory distress syndrome and avian influenza (H5N1) infection in Vietnamese children. J. Infect. Dis. 200, 510–515 (2009).
Nakajima, N. et al. The first autopsy case of pandemic influenza (A/H1N1pdm) virus infection in Japan: detection of a high copy number of the virus in type II alveolar epithelial cells by pathological and virological examination. Jpn J. Infect. Dis. 63, 67–71 (2010).
Mauad, T. et al. Lung pathology in fatal novel human influenza A (H1N1) infection. Am. J. Respir. Crit. Care Med. 181, 72–79 (2009).
Takeda, S. et al. Extracorporeal membrane oxygenation for 2009 influenza A(H1N1) severe respiratory failure in Japan. J. Anesth. 26, 650–657 (2012).
Beigel, J. & Bray, M. Current and future antiviral therapy of severe seasonal and avian influenza. Antiviral. Res. 78, 91–102 (2008).
Hayden, F. G. & Straus, S. E. Use of the oral neuraminidase inhibitor oseltamivir in experimental human influenza. JAMA 282, 1240–1246 (1999).
Stephenson, I. et al. Neuraminidase inhibitor resistance after oseltamivir treatment of acute influenza A and B in children. Clin. Infect. Dis. 48, 389–396 (2009).
Hurt, A. C., Holien, J. K., Parker, M., Kelso, A. & Barr, I. G. Zanamivir-resistant influenza viruses with a novel neuraminidase mutation. J. Virol. 83, 10366–10373 (2009).
Izumi, Y., Tokuda, K., O’Dell, K. A., Zorumski, C. F. & Narahashi, T. Synaptic and behavioral interactions of oseltamivir (Tamiflu) with neurostimulants. Hum. Exp. Toxicol. 27, 911–917 (2008).
Goldman, R. C. & Scaglione, F. The macrolide-bacterium interaction and its biological basis. Curr. Drug Targets Infect. Disord. 4, 241–260 (2004).
Tsurita, M. et al. Early augmentation of interleukin (IL)-12 level in the airway of mice administrated orally with clarithromycin or intranasally with IL-12 results in alleviation of influenza infection. J. Pharmacol. Exp. Ther. 298, 362–368 (2001).
Miyamoto, D. et al. Clarithromycin inhibits progeny virus production from human influenza virus-infected host cells. Biol. Pharm. Bull. 31, 217–222 (2008).
Yamaya, M. et al. Clarithromycin inhibits type A seasonal influenza virus infection in human airway epithelial cells. J. Pharmacol. Exp. Ther. 333, 81–90 (2010).
Ishii, H. et al. Clarithromycin has limited effects in non-elderly, non-severe patients with seasonal influenza virus A infection. J. Infect. 64, 343–345 (2012).
Hashimoto, Y., Moki, T., Takizawa, T., Shiratsuchi, A. & Nakanishi, Y. Evidence for phagocytosis of influenza virus-infected, apoptotic cells by neutrophils and macrophages in mice. J. Immunol. 178, 2448–2457 (2007).
Yamamoto, K., Miyoshi-Koshino, T., Utsuki, Y., Mizuno, S. & Suzuki, K. Virucidal activity and viral protein modification by myeloperoxidase: A candidate for defense factor of human polymorphonuclear leukocytes against influenza virus infection. J. Infect. Dis. 164, 8–14 (1991).
Klebanoff, S. J. Myeloperoxidase: friend and foe. J. Leukoc. Biol. 77, 598–625 (2005).
Bengtsson, T., Dahlgren, C., Stendahl, O. & Andersson, T. Actin assembly and regulation of neutrophil function: effects of cytochalasin B and retracaine on chemotactic peptide-induced O2− production and degranulation. J. Leuk. Biol. 49, 236–244 (1991).
Perrone, L. A., Plowden, J. K., Garcia-Sastre, A., Katz, J. M. & Tumpey, T. M. H5N1 and 1918 pandemic influenza virus infection results in early and excessive infiltration of macrophages and neutrophils in the lung of mice. PLoS Pathog. 4, e1000115 (2008).
Crowe, C. R. et al. Critical role of IL-17RA in immunopathology of influenza infection. J. Immunol. 183, 5301–5310 (2009).
Van, den. Brand, J. M. et al. Comparison of temporal and spatial dynamics of seasonal H3N2, pandemic H1N1 and highly pathogenic avian influenza H5N1 virus infections in ferrets. PLoS One 7, e42343 (2012).
Sugamata, R. et al. Contribution of neutrophil-derived myeloperoxidase in the early phase of fulminant acute respiratory distress syndrome induced by influenza virus infection. Microbiol. Immunol. 56, 171–182 (2012).
Okawara, A. I. et al. Purification and characterization of aseanostatins: actinomycete-derived fatty acid inhibitors to myeloperoxidase release from human polymorphonuclear leukocytes. J. Antibiot. 44, 524–532 (1991).
Ward, C. L. et al. Design and performance testing of quantitative real time PCR assays for influenza A and B viral load measurement. J. Clin. Virol. 29, 179–188 (2004).
Sugawara, A. et al. Novel 12-membered non-antibiotic macrolides from erythromycin A; EM900 series as novel leads for anti-inflammatory and/or immunomodulatory agents. Bioorg. Med. Chem. Lett. 21, 3373–3376 (2011).
Sugawara, A. et al. Novel 12-membered non-antibiotics macrolides, EM900 series with anti-inflammatory and/or immunomodulatory activity; synthesis, structure-activity relationships and in vivo study. J. Antibiot. 65, 487–490 (2012).
Schmitz, N., Kurrer, M., Bachmann, M. F. & Kopf, M. Interleukin-1 is responsible for acute lung immunopathology but increases survival of respiratory influenza virus infection. J. Virol. 79, 6441–6448 (2005).
Dienz, O. et al. Essential role of IL-6 in protection against H1N1 influenza virus by promoting neutrophil survival in the lung. Mucosal. Immunol. 5, 258–266 (2012).
Jesaitis, A. J., Tolley, J. O. & Allen, R. A. Receptor-cytoskeleton interactions and membrane traffic may regulate chemoattractant-induced superoxide production in human granulocytes. J. Biol. Chem. 261, 13662–13669 (1986).
Okawara, A. I. et al. Neutrophil contribution to the crescentic glomerulonephritis in SCG/Kj mice. Nephrol. Dial. Transplant. 19, 1708–1715 (2004).
Poste, G & Allison, A. C. Membrane fusion. Biochem. Biophys. Acta 300, 421–465 (1973).
Ryder, M. I., Weinreb, R. N. & Niederman, R. Microtubule-granule relationships in motile human polymorphonuclear leukocytes. Anat. Rec. 221, 679–686 (1988).
Tanaka, J. et al. Biomolecular mimicry in the actin cytoskeleton: mechanisms underlying the cytotoxicity of kabiramide C and related macrolides. Proc. Natl Acad. Sci. USA 25, 13851–13856 (2003).
Saito, S. et al. Novel actin depolymerizing macrolide aplyronine A. J. Biochem. 120, 552–555 (1996).
Ochiai, H., Sakai, S., Hirabayashi, T., Shimizu, Y & Terasawa, K. Inhibitory effect of bafilomycin A1, a specific inhibitor of vacuolar-type proton pump, on the growth of influenza A and B viruses in MDCK cells. Antiviral. Res. 27, 425–430 (1995).
Togashi, K., Kataoka, T. & Nagai, K. Characterization of a series of vacuolar type H+ -ATPase inhibitors on CTL-mediated cytotoxicity. Immunol. Lett. 55, 139–144 (1997).
Jang, Y. J., Kwon, H. -J. & Lee, B. -J. Effect of clarithromycin on rhinovirus-16 infection in A549 cells. Eur. Respir. J. 27, 12–19 (2006).
Morikawa, K., Zhang, J., Nonaka, M. & Morikawa, S. Modulatory effect of macrolide antibiotics on the Th1- and Th2-type cytokine production. Int. J. Antimicrob. Agents 19, 53–59 (2002).
Arimori, Y. et al. Type I interferon limits influenza virus-induced acute lung injury by regulation of excessive inflammatory in mice. Antiviral Res. 99, 230–237 (2013).
Salomon, R., Hoffmann, E. & Webster, R. G. Inhibition of the cytokine response does not protect against lethal H5N1 influenza infection. Proc. Natl Acad. Sci. USA 104, 12479–12481 (2007).
Peper, R. L. & Campen, H. Tumor necrosis factor as a mediator of inflammation in influenza A viral pneumonia. Microb. Pathog. 19, 175–183 (1995).
Kawaguchi, M. et al. Expression of eotaxin by normal airway epithelial cells after influenza virus A infection. Int. Arch. Allergy Immunol. 122 (suppl 1), 44–49 (2000).
Phung, T. T. et al. Key role of regulated upon activation normal T-cell expressed and secreted, nonstructural protein 1 and myeloperoxidase in cytokine storm induced by influenza virus PR-8 (A/H1N1) infection in A549 bronchial epithelial cells. Microbiol. Immunol. 55, 874–884 (2011).
Szretter, K. J. et al. Role of host cytokine responses in the pathogenesis of avian H5N1 influenza viruses in mice. J. Virol. 81, 2736–2744 (2007).
Roberts, N., Hiscott, J. & Signs, D. J. The limited role of the human interferon system response to respiratory syncytial virus challenge: analysis and comparison to influenza virus challenge. Microb. Pathog. 12, 409–414 (1992).
Barchet, W. et al. Dendritic cells respond to influenza virus though TLR7- and PKR-independent pathways. Eur. J. Immunol. 35, 236–242 (2005).
Domizio, J. D. et al. TLR7 stimulation in human plasmacytoid dendritic cells leads to the induction of early IFN-inducible genes in the absence of type I IFN. Blood 114, 1794–1802 (2009).
Asselin-Paturel, C. et al. Mouse type I IFN-producing cells are immature APCs with plasmacytoid morphology. Nat. Immunol. 2, 1144–1150 (2001).
Sieczkarski, S. B., Brown, H. B & Whittaker, G. R. Role of protein kinase C βII in influenza virus entry via late endosomes. J. Virol. 77, 460–469 (2003).
White, J., Kielian, M & Helenius, A. Membrane fusion proteins of enveloped animal viruses. Q. Rev. Biophys. 16, 151–195 (1983).
Ochiai, H., Sakai, S., Hirabayashi, T., Shimizu, Y & Terasawa, K. Inhibitory effect of bafilomycin A1, a specific inhibitor of vacuolar-type proton pump, on the growth of influenza A and B viruses in MDCK cells. Antiviral. Res. 27, 425–430 (1995).
Acknowledgements
This study was supported by a Research-in-Aid Grant from the Ministry of Health, Labor and Welfare of Japan (H22-S-I-014) and the Health Science Foundation of Japan (H21-004).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on The Journal of Antibiotics website
Rights and permissions
About this article
Cite this article
Sugamata, R., Sugawara, A., Nagao, T. et al. Leucomycin A3, a 16-membered macrolide antibiotic, inhibits influenza A virus infection and disease progression. J Antibiot 67, 213–222 (2014). https://doi.org/10.1038/ja.2013.132
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ja.2013.132
Keywords
This article is cited by
-
The effect of vitamin D, magnesium and zinc supplements on interferon signaling pathways and their relationship to control SARS-CoV-2 infection
Clinical and Molecular Allergy (2021)
-
The non-antibiotic macrolide EM900 attenuates HDM and poly(I:C)-induced airway inflammation with inhibition of macrophages in a mouse model
Inflammation Research (2020)
-
Azithromycin, a 15-membered macrolide antibiotic, inhibits influenza A(H1N1)pdm09 virus infection by interfering with virus internalization process
The Journal of Antibiotics (2019)
-
Triple combination of FDA-approved drugs including flufenamic acid, clarithromycin and zanamivir improves survival of severe influenza in mice
Archives of Virology (2018)
-
Blockade of programmed death-1/programmed death ligand pathway enhances the antitumor immunity of human invariant natural killer T cells
Cancer Immunology, Immunotherapy (2016)