Abstract
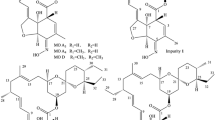
Analysis of commercial spectinomycin samples with ion-pairing reversed-phase LC coupled with electrospray ionization tandem MS (LC/ESI-MS/MS) indicates that eight additional compounds are present, including actinamine, (4R)-dihydrospectinomycin, (4S)-dihydrospectinomycin and dihydroxyspectinomycin, as well as four new impurities reported, to our knowledge, for the first time. The structures of these compounds were elucidated by comparing their fragmentation patterns with known structures, and NMR was employed to characterize and distinguish (4R)-dihydrospectinomycin and (4S)-dihydrospectinomycin. Identification of dihydrospectinomycin isomers is necessary because (4R)-dihydrospectinomycin is a minor active pharmaceutical ingredient of spectinomycin, whereas (4S)-dihydrospectinomycin is considered to be an impurity (impurity C) by the European Pharmacopoeia (Ph. Eur.).
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Spectam Oral Solution product information (Vetoquinol-Canada) (2007) Available at http://www.vetoquinol.ca.
Cuerpo, L & Livingston, R. C Residues of some veterinary drugs in animals and foods. Monographs prepared by the forty-second meeting of the joint FAO/WHO Expert Committee on Food Additives. FAO Food Nutr. Pap. 41, 1–86 (1994).
Ison, C. A Antimicrobial agents and gonorrhoea: therapeutic choice, resistance and susceptibility testing. Genitourin. Med. 72, 253–257 (1996).
Hoebus, J, Yun, L. M & Hoogmartens, J An improved gas chromatographic assay for spectinomycin hydrochloride. Chromatographia 39, 71–73 (1994).
Phillips, J. G & Simmonds, C Determination of spectinomycin using cation-exchange chromatography with pulsed amperometric detection. J. Chromatogr. A 675, 123–128 (1994).
Wang, M. J & Hu, C. Q Analysis of spectinomycin by high-performance liquid chromatography with evaporative light-scattering detection. Chromatographia 63, 255–260 (2006).
The European Pharmacopoeia Commission. The European Pharmacopoeia 7th edn. 2969–2971 Council of Europe: Strasbourg, (2011).
Basak, A. K, Raw, A. S & Yu, L. X Pharmaceutical impurities: analytical, toxicological and regulatory perspectives. Adv. Drug. Deliver. Rev. 59, 1–2 (2007).
Ahuja, S Assuring quality of drugs by monitoring impurities. Adv. Drug. Deliver. Rev. 59, 3–11 (2007).
Hu, C. Q Current situation and the trend in impurity control of chemical drugs. Sci. China-Chem. 40, 679–687 (2010).
ICH harmonised tripartite guideline. Q3A (R2): Impurities in New Drug Substances (Current Step 4 version, dated 25 October 2006). Available at http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q3A_R2/Step4/Q3A_R2__Guideline.pdf.
Vogel, R, Defillipo, K & Reif, V Determination of isepamicin sulfate and related compounds by high performance liquid chromatography using evaporative light scattering detection. J. Pharm. Biomed. Anal. 24, 405–412 (2001).
Megoulas, N. C & Koupparis, M. A Enhancement of evaporative light scattering detection in high-performance liquid chromatographic determination of neomycin based on highly volatile mobile phase, high-molecular-mass ion-pairing reagents and controlled peak shape. J. Chromatogr. A 1057, 125–131 (2004).
Clarot, I et al. Simultaneous quantitation of tobramycin and colistin sulphate by HPLC with evaporative light scattering detection. J. Pharm. Biomed. Anal. 50, 64–67 (2009).
Hong, JW, Hu, C. Q & Sheng, L. S Analysis of the response factors of different quinolones detected by evaporative light-scattering detector. Acta Pharm. Sin. 38, 695–697 (2003).
Sarri, AK, Megoulas, N. C & Koupparis, M. A Development of a novel liquid chromatography-evaporative light scattering detection method for bacitracins and applications to quality control of pharmaceuticals. Anal. Chim. Acta 28, 250–257 (2006).
Megoulas, NC & Koupparis, M. A Development and validation of a novel LC/ELSD method for the quantitation of gentamicin sulfate components in pharmaceuticals. J. Pharm. Biomed. Anal. 36, 73–79 (2004).
Young, CS & Dolan, J. W Success with evaporative light-scattering detection, part II: tips and techniques. LC-GC Eur. 17, 192–201 (2004).
Zhou, JY, Zhang, L, Wang, Y & Yan, C HPLC-ELSD analysis of spectinomycin dihydrochloride and its impurities. J. Sep. Sci. 34, 1811–1819 (2011).
Debremaeker, D et al. Analysis of spectinomycin by liquid chromatography with pulsed electrochemical detection. J. Chromatogr. A 953, 123–132 (2002).
Prčetić, K. V, Cservenák, R & Radulović, N Development and validation of liquid chromatography tandem mass spectrometry methods for the determination of gentamicin, lincomycin, and spectinomycin in the presence of their impurities in pharmaceutical formulations. J. Pharm. Biomed. Anal. 56, 736–742 (2011).
Takeda, N, Harada, K, Suzuki, M, Tatematsu, A & Kubodera, T Application of emitter chemical ionization mass spectrometry to structural characterization of aminoglycoside antibiotics–2. Org. Mass Spectrom. 17, 247–252 (1982).
Kotretsou, S. I & Kokotou, V. C Mass spectrometric studies on the fragmentation and structural characterization of aminoacyl derivatives of kanamycin A. Carbohydr. Res. 310, 121–127 (1998).
Goolsby, B. J & Brodbelt, J. S Analysis of protonated and alkali metal cationized aminoglycoside antibiotics by collision-activated dissociation and infrared multi-photon dissociation in the quadrupole ion trap. J. Mass Spectrom. 35, 1011–1024 (2000).
Hu, P. F Collisionally activated dissociations of aminocyclitol-aminoglycoside antibiotics and their application in the identification of a new compound in tobramycin samples. J. Am. Soc. Mass Spectrom. 11, 200–209 (2000).
McLaughlin, L. G & Henion, J. D Determination of aminoglycoside antibiotics by reversed-phase ion-pair high-performance liquid chromatography coupled with pulsed amperometry and ion spray mass spectrometry. J. Chromatogr. 591, 195–206 (1992).
Wang, J, Hu, X. J, Tu, Y & Ni, K. Y Determination of spectinomycin hydrochloride and its related substances by HPLC-ELSD and HPLC-MSn. J. Chromatogr. B 834, 178–182 (2006).
Antibiotics-column Selection Recommendation Database (accessed: 1 June 2013). Available at http://www.antibiotic.cn/columnt.htm.
Carey, F. A Organic Chemistry. (McGraw Hill Higher Education, New Jersey, (2007).
Acknowledgements
This article was supported by the Youth Development Research Foundation of NIFDC (Project No. 7030230111102). We acknowledge Yi Wang from Ge Rui High Technology (Hebei, China) for preparation of dihydrospectinomycin, Yaozuo Yuan from the Jiangsu Institute for Food and Drug Control (Jiangsu, China) for providing help in the fragmentation pattern of spectinomycin and Erwin Adams from KU Leuven for many helpful suggestions on the modification of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on The Journal of Antibiotics website
Rights and permissions
About this article
Cite this article
Wang, Y., Wang, M., Li, J. et al. Characterization of impurities in commercial spectinomycin by liquid chromatography with electrospray ionization tandem mass spectrometry. J Antibiot 67, 511–518 (2014). https://doi.org/10.1038/ja.2014.32
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ja.2014.32
Keywords
This article is cited by
-
Solubility Measurement, Model Correlation, and Solvent Effect Analysis of Spectinomycin Dihydrochloride Pentahydrate in Three Binary Solvents
Journal of Solution Chemistry (2024)
-
Anaerobic digestion of spectinomycin mycelial residues pretreated by thermal hydrolysis: removal of spectinomycin and enhancement of biogas production
Environmental Science and Pollution Research (2020)