Abstract
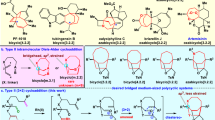
Synthesis of a cis-decalin moiety, containing an oxa-bridged cis-decalin ring system (11-oxatricyclo(5.3.1.1,703,8)undecane), as a key intermediate of the total synthesis of luminamicin (1) was accomplished. One of the essential steps in our synthetic route is construction of a cis-decaline framework using a one-pot Michael addition-aldol reaction. Additionally, the bridged ether moiety was obtained by an intramolecular 1,6-oxa-Michael reaction of a conjugated aldehyde.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Shah, D. et al. Clostridium difficile infection: update on emerging antibiotic treatment options and antibiotic resistance. Expert Rev. Anti. Infect. Ther. 8, 555–564 (2010).
Leffler, D. A. & Lamont, J. T. Clostridium difficile infection. N. Engl. J. Med. 372, 1539–1548 (2015).
Ōmura, S. et al. Luminamicin, a new antibiotic: production, isolation and physico-chemical and biological properties. J. Antibiot. 38, 1322–1326 (1985).
Jackson, M. et al. Coloradocin, an antibiotic from a new actinoplanes I. taxonomy, fermentation and biological properties. J. Antibiot. 40, 1375–1382 (1987).
Rasmussen, R. R., Scherr, M. H., Whittern, D. N., Buko, A. M. & McAlpine, J. B. Coloradocin, an antibiotic from a new actinoplanes II. Identity with luminamicin and elucidation of structure. J. Antibiot. 40, 1383–1393 (1987).
Whaley, H. A., Chindester, C. G., Mizsak, S. A. & Wnuk, R. J. Nodusmicin: the structure of a new antibiotic. Tetrahedron Lett. 21, 3659–3662 (1980).
Celmer, W. D. et al. Structure of natural antibiotic CP-47,444. J. Am. Chem. Soc. 102, 4203–4209 (1980).
Sohng, J. K. et al. Production, isolation and biological activity of nargenicin from Nocardia sp. CS682. Arch. Pharm. Res. 31, 1339–1345 (2008).
Gouda, H. et al. Stereostructure of luminamicin, an anaerobic antibiotic, via molecular dynamics, NMR spectroscopy, and the modified Mosher method. Proc. Natl. Acad. Sci. USA 102, 18286–18291 (2005).
Kimishima, A. et al. Toward the total synthesis of luminamicin: construction of 14-membered lactone framework possessing versatile enol ether moiety. Tetrahedron Lett. 53, 2813–2816 (2012).
Plata, D. J. & Kallmerten, J. Total synthesis of (+)-18-deoxynargenicin A1 . J. Am. Chem. Soc. 110, 4041–4042 (1988).
Marchart, S., Gromov, A. & Mulzer, J. Total synthesis of the antibiotic branimycin. Angew. Chem. Int. Ed. 49, 2050–2053 (2010).
Enev, V. S., Felzmann, W., Gromov, A., Marchart, S. & Mulzer, J. Total synthesis of branimycin: an evolutionary approach. Chem. Eur. J. 18, 9651–9668 (2012).
Čikos, A. et al. Reinvestigation of the branimycin stereochemistry at position 17-C. Org. Lett. 18, 780–783 (2016).
Sunazuka, T. et al. Synthesis of the oxa-bridged octalin system of two anti-anaerobe antibiotics, luminamicin and lustromycin. Tetrahedron Lett. 48, 5297–5300 (2007).
Nicolaou, K. C., Brenzovich, W. E., Bulgera, P. G. & Francisa, T. M. Synthesis of iso-epoxy-amphidinolide N and des-epoxy-caribenolide I structures. Initial forays. Org. Biomol. Chem. 4, 2119–2157 (2006).
Wullschleger, C. W., Li, J., Edenharter, A. & Altmann, K.-H. Studies towards the synthesis of leiodolide A. Synlett 27, 2726–2730 (2016).
Boulet, S. L. & Paquette, L. A. Toward a total synthesis of okilactomycin. 2. A metathesis-based approach to the heavily functionalized cyclohexane ring. Synthesis 7, 895–900 (2002).
Rychnovsky, S. D., Rogers, B. & Yang, G. Analysis of two 13C NMR correlations for determining the stereochemistry of 1,3-diol acetonides. J. Org. Chem. 58, 3511–3515 (1993).
Uchida, K., Ishigami, K., Watanabe, H. & Kitahara, T. Synthesis of an insecticidal tetrahydroisocoumarin, (3R,4S,4aR-4,8-dihydroxy-3-methyl-3,4,4a,5-tetrahydro-1H-2-benzopyran-1-one. Tetrahedron 63, 1281–1287 (2007).
Dias, L. C., Melgar, G. Z. & Jardim, L. S. A. A short approach to the bicyclo[4.3.0]nonane fragment of stawamycin. Tetrahedron Lett. 46, 4427–4431 (2005).
Acknowledgements
This work was supported by Japan Society for the Promotion of Science (JSPS) KAKENHI Grants (numbers 16K08175, 26860015, 24790022 and 22890175) (AS); a Meiji Seika Pharma Award in Synthetic Organic Chemistry Japan (AS); a Kitasato University Research Grant for Young Researchers (AS), JSPS Research Fellowships for Young Scientists (TM and AK) and a Uehara Memorial foundation grant (TH). We also thank Dr K Nagai and Ms N Sato (School of Pharmacy, Kitasato University) for their analyses of mass and NMR spectra.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Dedicated to Professor KC Nicolaou and his outstanding contributions to complex natural product total synthesis and chemical biology.
Supplementary Information accompanies the paper on The Journal of Antibiotics website
Supplementary information
Rights and permissions
About this article
Cite this article
Ando, H., Kimishima, A., Ohara, M. et al. Toward the total synthesis of luminamicin; an anaerobic antibiotic: construction of highly functionalized cis-decalin containing a bridged ether moiety. J Antibiot 71, 268–272 (2018). https://doi.org/10.1038/ja.2017.77
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/ja.2017.77