Abstract
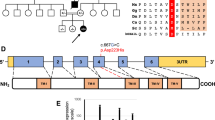
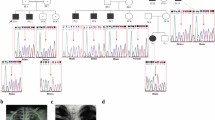
We applied whole-exome sequencing (WES) for identification of an underlying genetic cause of a disease in a family presented with fatal infantile hyperthermia. Analysis of WES results revealed novel, deleterious compound missense mutations, Val160Ala and Pro233Thr, in the synthesis of cytochrome C oxidase 2 gene (SCO2) encoding a mitochondrial protein, Sco2, which is important for cytochrome C oxidase (COX) synthesis. Autosomal recessive mutations in SCO2 are known to be associated with COX deficiency recognized as fatal infantile cardio-encephalomyopathy (604272, OMIM). The Val160Ala and Pro233Thr mutations occurred in the conserved thioredoxin domain of Sco2 and predicted to disrupt protein folding and interaction of Sco2 with other proteins. Our results show applicability of WES in identification of disease-causing mutations and in establishing molecular diagnosis of severe, infantile onset disorder with a challenging diagnosis.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Bamshad, M. J., Ng, S. B., Bigham, A. W., Tabor, H. K., Emond, M. J., Nickerson, D. A. et al. Exome sequencing as a tool for Mendelian disease gene discovery. Nat. Rev. Genet. 12, 745–755 (2011).
Majewski, J., Schwartzentruber, J., Lalonde, E., Montpetit, A. & Jabado, N. What exome sequencing can do for you? J. Med. Genet. 48, 580–589 (2011).
Papadopoulou, L. C., Sue, C. M., Davidson, M. M., Tanji, K., Nishino, I., Sadlock, E. J. et al. Fatal infantile cardioencephalomyopathy with COX deficiency and mutations in SCO2, a COX assembly gene. Nat. Genet. 23, 333–337 (1999).
Leary, S. C., Sasarman, F., Nishimura, T. & Shoubridge, E. A. Human SCO2 is required for the synthesis of CO II and as a thiol-disulphide oxidoreductase for SCO1. Hum. Mol. Genet. 18, 2230–2240 (2010).
Verdijk, R. M., de Krijger, R., Schoonderwood, K., Tiranti, V., Smeets, H., Govaerts, L. C. P. et al. Phenotypic consequences of a novel SCO2 gene mutation. Am. J. Med. Genet. 146, 2822–2827 (2008).
Joost, K., Rodenburg, R., Piirsoo, A., van den Heuvel, B., Zordania, R. & Ōunap, K. A novel mutation in the SCO2 gene in a neonate with early-onset cardioencephalomyopathy. Pediatr. Neurol. 42, 227–230 (2010).
Pronicki, M., Kowalski, P., Piekutowska-Abramczuk, D., Taybert, J., Karkucinska-Wieskowska, A., Szymanska-Debinska, T. et al. A homozygous mutation in the SCO2 gene causes a spinal muscular atrophy like presentation with stridor and respiratory insufficiency. Eur. J. Pediatr. Neurol. 14, 253–260 (2010).
Groom, L., Muldoon, S., Tang, Z., Brandom, B. W., Bayarsaikhan, M., Bina, S. et al. Identical de novo mutation in the RYR1 gene associated with fatal, stress-induced malignant hyperthermia in two unrelated families. Anesthesiology 115, 938–945 (2011).
Rosenberg, H., Sambuughin, N. & Dirksen, R. T. Malignant hyperthermia susceptibility in GeneReviews at GeneTests: Medical Genetics Information Resource [database online]. Copyright, University of Washington, Seattle 1997–2012. Available at http://www.genetests.org.
Robinson, R. L., Carpenter, D., Shaw, M., Halsall, J. & Hopkins, P. Mutations in RYR1 in Malignant hyperthermia and central core disease. Hum. Mutat. 27, 977–989 (2006).
Tobin, J., Jason, D. R., Challa, V. R., Nelson, T. E. & Sambuughin, N. Malignant hyperthermia and apparent heat stroke. JAMA 286, 168–169 (2001).
Consortium TGP. A map of human genome variation from population scale sequencing. Nature 467, 1061–1073 (2010).
NHLBI Exome Sequencing Project. Seattle, WA, URL: http://evs.gs.washington.edu/EVS/. Accessed 02 July 2012.
Bianci, L., Bertini, I., Ciofi-Baffoni, S., Gerothanassis, I. P., Leontari, I., Martnelli, M. et al. A structural characterization of human SCO2. Structure 15, 1132–1140 (2007).
Sacconi, S., Salviati, L., Sue, C. M., Shanske, S., Davidson, M. M., Bonilla, E. et al. Mutation screening in patients with isolated cytochrome c oxidase deficiency. Pediatr. Res. 53, 224–230 (2003).
Figarella-Branger, D., Kozak-Ribbens, G., Rodet, L., Aubert, M., Borsarelli, J., Cozzone, P. J. et al. Pathological findings in 165 patients explored for malignant hyperthermia susceptibility. Neuromusc. Disord. 3, 553–556 (1993).
Finsterer, J., Michalek-Sauberer, A. & Hoftberger, R. Malignant hyperthermia susceptibility in a patient with mitochondrial disorder. Metab. Brain Dis. 24, 501–506 (2009).
Ross, A. K. Muscular dystrophy versus mitochondrial myopathy: the dilemma of the undiagnosed hypotonic child. Pediatr. Anesth. 17, 1–6 (2007).
Knuff, M., Faber, J., Huth, R. G., Freisinger, P., Zepp, F. & Kampmann, C. Identification of a novel compound heterozygote SCO2 mutation in cytochrome c oxidase deficient fatal infantile cardiomyopathy. Acta. Pediatrica. 96, 128–134 (2007).
Mobely, B. C., Enns, G. M., Wong, L. J. & Vogel, H. A novel homozygous SCO2 mutation, p.G193S, causing fatal infantile cardioencephalomyopathy. Clin. Neuropathol. 28, 143–149 (2009).
Acknowledgements
We are grateful to the family for their participation in this study. We thank Dr W Watson, Department of Neurology, Uniformed Services University for valuable comments. The authors thank Biomedical Instrumentation Center of Uniformed Services University for oligo synthesis and Sanger sequencing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on Journal of Human Genetics website
Supplementary information
Rights and permissions
About this article
Cite this article
Sambuughin, N., Liu, X., Bijarnia, S. et al. Exome sequencing reveals SCO2 mutations in a family presented with fatal infantile hyperthermia. J Hum Genet 58, 226–228 (2013). https://doi.org/10.1038/jhg.2012.156
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/jhg.2012.156
Keywords
This article is cited by
-
A mutation in the TMEM65 gene results in mitochondrial myopathy with severe neurological manifestations
European Journal of Human Genetics (2017)