Abstract
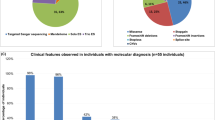
Recently, we defined a minimal overlapping region for causal Xp11.22 copy number gains in males with intellectual disability (ID), and identified HECT, UBA and WWE domain-containing protein-1 (HUWE1) as the primary dosage-sensitive gene, whose overexpression leads to ID. In the present study, we used this minimal interval to search for HUWE1 copy number variations by quantitative polymerase chain reaction in a large cohort of Brazilian males with idiopathic ID. We detected two unrelated sporadic individuals with syndromic ID carrying unique overlapping duplications encompassing HUWE1. Breakpoint junction analysis showed a simple tandem duplication in the first patient, which has probably arisen by microhomology-mediated break-induced repair mechanism. In the second patient, the rearrangement is complex having an insertion of an intrachromosomal sequence at its junction. This kind of rearrangement has not been reported in Xp11.22 duplications and might have emerged by a replication- or recombination-based mechanism. Furthermore, the presence of infantile seizures in the second family suggests a potential role of increased KDM5C expression on epilepsy. Our findings highlight the importance of microduplications at Xp11.22 to ID, even in sporadic cases, and reveal new clinical and molecular insight into HUWE1 copy number gains.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Froyen, G., Corbett, M., Vandewalle, J., Jarvela, I., Lawrence, O. & Meldrum, C. et al. Submicroscopic duplications of the hydroxysteroid dehydrogenase HSD17B10 and the E3 ubiquitin ligase HUWE1 are associated with mental retardation. Am. J. Hum. Genet. 82, 432–443 (2008).
Froyen, G., Belet, S., Martinez, F., Santos-Rebouças, C. B., Declercq, M. & Verbeeck, J. et al. Copy-number gains of HUWE1 due to replication- and recombination-based rearrangements. Am. J. Hum. Genet. 91, 252–264 (2012).
Van Esch, H., Bauters, M., Ignatius, J., Jansen, M., Raynaud, M. & Hollanders, K. et al. Duplication of the MECP2 region is a frequent cause of severe mental retardation and progressive neurological symptoms in males. Am. J. Hum. Genet. 77, 442–453 (2005).
Santos-Rebouças, C. B., Belet, S., Guedes de Almeida, L., Ribeiro, M. G., Medina-Acosta, E. & Bahia, P. R. et al. A novel in-frame deletion affecting the BAR domain of OPHN1 in a family with intellectual disability and hippocampal alterations. Eur. J. Hum. Genet 22, 644–651 (2014).
Tahiliani, M., Mei, P., Fang, R., Leonor, T., Rutenberg, M. & Shimizu, F. et al. The histone H3K4 demethylase SMCX links REST target genes to X-linked mental retardation. Nature 447, 601–605 (2007).
Gonçalves, T. F., Gonçalves, A. P., Fintelman Rodrigues, N., Dos Santos, J. M., Pimentel, M. M. & Santos-Rebouças, C. B. KDM5C mutational screening among males with intellectual disability suggestive of X-Linked inheritance and review of the literature. Eur. J. Med. Genet. 57, 138–144 (2014).
Poeta, L., Fusco, F., Drongitis, D., Shoubridge, C., Manganelli, G. & Filosa, S. et al. A regulatory path associated with X-linked intellectual disability and epilepsy links KDM5C to the polyalanine expansions in ARX. Am. J. Hum. Genet. 92, 114–125 (2013).
Verdin, H., D'haene, B., Beysen, D., Novikova, Y., Menten, B. & Sante, T. et al. Microhomology-mediated mechanisms underlie non-recurrent disease-causing microdeletions of the FOXL2 gene or its regulatory domain. PLoS Genet. 9, e1003358 (2013).
Carvalho, C. M., Pehlivan, D., Ramocki, M. B., Fang, P., Alleva, B. & Franco, L. et al. Replicative mechanisms for CNV formation are error prone. Nat. Genet. 45, 1319–1326 (2013).
Acknowledgements
We thank the families for their kind cooperation and Centro Estadual de Diagnóstico por Imagem (SES, Rio de Janeiro, Brazil) for conducting the neuroimaging tests. This work was supported by funds from Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ/Brazil), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq/Brazil), CEPUERJ and the Geconcerteerde Onderzoeks Acties of the University of Leuven, Belgium (GOA/12/015).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Journal of Human Genetics website
Rights and permissions
About this article
Cite this article
Santos-Rebouças, C., de Almeida, L., Belet, S. et al. Novel microduplications at Xp11.22 including HUWE1: clinical and molecular insights into these genomic rearrangements associated with intellectual disability. J Hum Genet 60, 207–211 (2015). https://doi.org/10.1038/jhg.2015.1
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/jhg.2015.1
This article is cited by
-
Roles of the HUWE1 ubiquitin ligase in nervous system development, function and disease
Neural Development (2020)
-
Xp11.22 duplications in four unrelated Chinese families: delineating the genotype-phenotype relationship for HSD17B10 and FGD1
BMC Medical Genomics (2020)
-
What microRNAs could tell us about the human X chromosome
Cellular and Molecular Life Sciences (2020)
-
Network Profiling of Brain-Expressed X-Chromosomal MicroRNA Genes Implicates Shared Key MicroRNAs in Intellectual Disability
Journal of Molecular Neuroscience (2019)
-
Impaired oxidative stress response characterizes HUWE1-promoted X-linked intellectual disability
Scientific Reports (2017)