Abstract
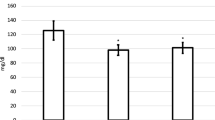
Niacin is the most effective drug available for raising levels of high-density lipoprotein (HDL) cholesterol. To evaluate its effects on plasma lipid concentrations, the authors administered a low dose of niacin to healthy, adult, female African green monkeys for 3 months. In the treated monkeys, low-density lipoprotein cholesterol concentrations decreased by 43% from baseline, whereas concentrations of HDL cholesterol and apolipoprotein A-I increased by 49% and 34%, respectively. The results suggest that in this primate model, a low dose of niacin can effectively increase concentrations of HDL cholesterol.
This is a preview of subscription content, access via your institution
Access options

Similar content being viewed by others
References
Vilahur, G., Padro, T. & Badimon, L. Atherosclerosis and thrombosis: insights from large animal models. J. Biomed. Biotechnol. 2011, 907575 (2011).
Moghadasian, M.H., Frohlich, J.J. & McManus, B.M. Advances in experimental dyslipidemia and atherosclerosis. Lab. Invest. 81, 1173–1183 (2001).
Ramharack, R., Bocan, T.M.A., Imperiale, M.J. & Spahr, M.A. Recombinant adenovirus vector mediated expression of lipoprotein (a) [Lp(a)] in rabbit plasma. Biochim. Biophys. Acta 1438, 322–328 (1999).
Yin, W. et al. Plasma lipid profiling across species for the identification of optimal animal models of human dyslipidemia. J. Lipid Res. 53, 51–65 (2012).
Moghadasian, M.H. Experimental atherosclerosis: a historical overview. Life Sci. 70, 855–865 (2002).
Carlsson, H., Schapiro, S.J., Farah, I. & Hau, J. Use of primates in research: a global overview. Am. J. Primatol. 63, 225–237 (2004).
Cefalu, W.T. & Wagner, J.D. Aging and atherosclerosis in human and nonhuman primates. Age 20, 15–28 (1997).
Weight, M.J. et al. Low density lipoprotein kinetics in African Green monkeys showing variable cholesterolaemic responses to diets realistic for westernised people. Atherosclerosis 73, 1–11 (1988).
Suckling, K.E. & Jackson, B. Animal models of human lipid metabolism. Prog. Lipid Res. 32, 1–24 (1993).
Bullock, B.C. et al. Comparative primate atherosclerosis: I. Tissue cholesterol concentration and pathologic anatomy. Exp. Mol. Pathol. 22, 151–175 (1975).
Fincham, J.E. et al. Diets realistic for westernised people significantly affect lipoproteins, calcium, zinc, vitamins C, E, B6 and haematology in Vervet monkeys. Atherosclerosis 66, 191–203 (1987).
Nichols, A.V. & Smith, L. Effect of very low-density lipoproteins on lipid transfer in incubated serum. J. Lipid Res. 6, 206–210 (1965).
Carroll, R.M. & Rudel, L.L. Dietary fat and cholesterol effects on lipoprotein cholesterol ester formation via lecithin:cholesterol acyltransferase (LCAT) in vervet monkeys. Fed. Proc. 40, 1695 (1981).
Fernandez, M. & Wood, R. in Sourcebook of Models for Biomedical Research (ed. Conn, P.M.) 201–212 (Humana, Totowa, NJ, 2008).
Carlson, L.A. Nicotinic acid: the broad-spectrum lipid drug. A 50th anniversary review. J. Intern. Med. 258, 94–114 (2005).
Ohashi, R., Mu, H., Wang, X., Yao, Q. & Chen, C. Reverse cholesterol transport and cholesterol efflux in atherosclerosis. QJM 98, 845–856 (2005).
Rader, D.J. Regulation of reverse cholesterol transport and clinical implications. Am. J. Cardiol. 92, 42–49 (2003).
Kamanna, V.S., Ganji, S.H. & Kashyap, M.L. The mechanism and mitigation of niacin-induced flushing. Intl. J. Clin. Pract. 63, 1369–1377 (2009).
Kamanna, V.S. & Kashyap, M.L. Mechanism of action of niacin. Am. J. Cardiol. 101, S20–S26 (2008).
Zhang, L.-H., Kamanna, V.S., Zhang, M.C. & Kashyap, M.L. Niacin inhibits surface expression of ATP synthase β chain in HepG2 cells: implications for raising HDL. J. Lipid Res. 49, 1195–1201 (2008).
Seier, J.V. Breeding vervet monkeys in a closed environment. J. Med. Primatol. 15, 339–349 (1986).
Venter, F.S., Cloete, H., Seier, J.V., Faber, M. & Fincham, J.E. Folic acid and vitamin B12 status of vervet monkeys used for nutritional research. Lab. Anim. 27, 59–64 (1993).
Tavintharan, S. & Kashyap, M. The benefits of niacin in atherosclerosis. Curr. Atheroscler. Rep. 3, 74–82 (2001).
Shamekh, R. et al. Endogenous and diet-induced hypercholesterolemia in nonhuman primates: effects of age, adiposity, and diabetes on lipoprotein profiles. Metabolism 60, 1165–1177 (2011).
Fusegawa, Y., Kelley, K.L., Sawyer, J.K., Shah, R.N. & Rudel, L.L. Influence of dietary fatty acid composition on the relationship between CETP activity and plasma lipoproteins in monkeys. J. Lipid Res. 42, 1849–1857 (2001).
Wallace, J.M. et al. Effects of peroxisome proliferator-activated receptor α/δ agonists on HDL-cholesterol in vervet monkeys. J. Lipid Res. 46, 1009–1016 (2005).
Offermanns, S. The nicotinic acid receptor GPR109A (HM74A or PUMA-G) as a new therapeutic target. Trends Pharmacol. Sci. 27, 384–390 (2006).
Kullo, I.J., Jan, M.F., Bailey, K.R., Mosley, T.H. & Turner, S.T. Ethnic differences in low-density lipoprotein particle size in hypertensive adults. J. Clin. Lipidol. 1, 218–224 (2007).
Rudel, L.L., Parks, J.S. & Sawyer, J.K. Compared with dietary monounsaturated and saturated fat, polyunsaturated fat protects African green monkeys from coronary artery atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 15, 2101–2110 (1995).
Acknowledgements
This study was supported by the Primate Unit of the South African Medical Research Council. We thank Joritha van Heerden, Timothy Collop and Abraham Davids for their excellent technical assistance and expertise in primate management.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Chauke, C., Arieff, Z., Kaur, M. et al. Effects of short-term niacin treatment on plasma lipoprotein concentrations in African green monkeys (Chlorocebus aethiops). Lab Anim 43, 58–62 (2014). https://doi.org/10.1038/laban.424
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/laban.424