Abstract
Serous carcinoma (SC) represents ~10% of endometrial carcinomas, but is responsible for almost 40% of cancer deaths. This article reviews the main pathological features, differential diagnosis, and the usefulness of molecular pathology and immunohistochemistry in its diagnosis. Most helpful features for the diagnosis include: irregularly shaped and sized papillae, slit-like spaces, cell stratification and budding, highly atypical cells, architectural and cytological discordance in pseudoglandular tumors, as well as lack of endometrioid features. SC shows typically a predominant papillary growth, which is also found in some subtypes of endometrioid carcinoma of the endometrium (EEC). Distinction is easy when attention is paid to the presence of diffuse marked nuclear pleomorphism, but also to the complex papillary architecture. SC may also show a solid or pseudoglandular patterns, and in these cases differential diagnosis may be difficult with EEC grade 3. Moreover, a high proportion of SC may exhibit clear cells, and, thus, may be confused with clear cell carcinoma. Finally, it is sometimes difficult to distinguish mixed SC-EEC, from SC that combines papillary and pseudoglandular growths. Although there is not a single immunohistochemical marker for distinguishing SC from its mimickers, some antibodies are useful (p53, p16, IMP2, and IMP3), particularly when used in combination. Diagnosis of SC may be even more problematic in small biopsies; a diagnosis of high-grade endometrial carcinoma, SC component can not be excluded, is acceptable as a managerial approach, so it could be taken into account at the time of final surgery.
Similar content being viewed by others
Main
Serous carcinoma (SC) is the prototype of type-II endometrial cancer and accounts for <10% of all endometrial carcinomas (EC).1 It is a very aggressive tumor, unrelated to estrogen stimulation, arising occasionally in endometrial polyps or from precancerous lesions developing in atrophic endometrium that mainly occur in older women.2 Patients with SC are at median of 5–10 years older than those with endometrioid carcinomas of the endometrium (EEC). SCs have also been reported in association with a history of breast cancer. A possible role for tamoxifen, as well as BRCA1 and BRCA2 germline mutations has been proposed, but it remains unclear whether they are key players in the link between SC and breast cancer.
SC is, by definition, regarded as high-grade tumor. The term high-risk EC has been used to include SC, clear cell carcinoma (CC), and grade 3 EEC (EEC3), because of the poor prognosis associated with these three tumor types. SC has a high tendency to develop lymph node metastasis as well as adnexal and peritoneal spread, CC is also associated with nodal metastasis, whereas EEC3 metastasizes primarily to pelvic and para-aortic lymph nodes. These three types of EC comprise ~25–30% of EC, but account for 70–75% of EC deaths. SC and EEC3 have been compared using the Surveillance, Epidemiology and End Results program data from 1988 to 2001. They represented 10% and 15% of EC, respectively, but accounted for 39% and 27% of cancer deaths, respectively. SC is usually diagnosed at advanced stage; 46% of patients are at stage II–IV at presentation.3, 4, 5 The estimated 5-year overall survival for women with SC, CC, and EEC3 is 55%, 68% and 77%, respectivly. Approximately, 60–70% of women with SC present with disease outside the uterus.
SC is defined in WHO 2014 as having complex papillary and/or glandular arrangements, with diffuse marked nuclear pleomorphism.6 The typical microscopic features and its aggressive behavior were well described by Stanford group.7 However, in 1974, Factor had already described the existence of papillary adenocarcinomas of the endometrium with psammoma bodies, noting their similarity to ovarian cancer.8 In 1981, Lauchlan designated the tumors as ‘tubal’ or ‘serous’,9 and in 1994, SC was first included in the classification of endometrial carcinomas, by WHO.
Pathologic features
The gross appearance of SC is similar to that of EEC. The uterus is usually enlarged, but occasionally small and atrophic (Figure 1). Tumors are typically large, bulky, with one or several nodules protruding into the endometrial cavity, with frequent necrosis. There is often deep myometrial invasion. However, the tumor may be inconspicuous, restricted to the lining of an otherwise conventional endometrial polyp.
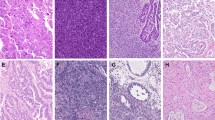
SC has usually, but not always, a prominent papillary pattern (Figure 2). In these cases, the tumor shows complex, thick, fibrotic, or edematous papillae with prominent stratification of tumor cells and cellular budding.10, 11, 12, 13, 14 The papillae are lined by pleomorphic cells with large, eosinophilic cytoplasm, with striking cellular pseudostratification, micropapillary formation, and cell budding (Figure 3). Occasionally, groups of detached tumor cells may be present lying between papillae. Tumor cells are cuboidal with eosinophilic cytoplasm, but focal clearing may be noted. The nuclei are hyperchromatic or vesicular, sometimes lobulated, and display prominent macronucleoli. Over one-third of SCs contain clear cells (Figure 4a) and cells with hobnail configuration (Figure 4b). In some areas, the tumor may show a solid pattern, with highly atypical cells (Figure 5), and in others a glandular arrangement with slit-like and irregular spaces (Figure 6). The solid- and glandular-like arrangements frequently coexist with the typical papillary pattern, but may be predominant or exclusive. Mitoses are frequent, and many of them are abnormal. Although SC typically contains highly atypical cells, some tumors may show deceptively bland nuclei. Psammoma bodies may be present, but less frequently than in SC of the fallopian tube/ovary. The tumor is usually associated with deep myometrial invasion and extensive lymphatic invasion. When there is myometrial invasion, a ‘gaping gland’ appearance often unaccompanied by stromal reaction is frequently seen. Transition between SC and non-neoplastic endometrium is sometimes abrupt, but in many cases non-invasive tumor cells may replace endometrial surface epithelium/glands, without invading the myometrium (Figure 7).
Serous carcinoma with clear cells (a) and hobnail cells (b). Serous carcinoma occasionally contains cells with clear cytoplasm or hobnail configuration, posing problems in differential diagnosis with clear cell carcinoma. Cell pseudostratification, high mitotic index and coexistence with typical papillary growth favors the diagnosis of serous carcinoma.
SC typically does not arise from pre-existing endometrial hyperplasia. A precursor lesion in different stages of evolution has been described, from p53 signature to endometrial glandular dysplasia.15, 16 These lesions usually develop in the setting of atrophic endometrium or endometrial polyps. p53 is one of the molecular abnormalities involved in the development and progression of this tumor; and tumor cells usually are strongly positive for p53 immunostaining. Like for SCs of the fallopian tube/ovary, the p53 signature is characterized by clusters of ‘normal-appearing’ cells with strong p53 immunostaining. Endometrial glandular dysplasia has also been suggested as a putative precursor of SC, based on histologic similarities, p53 alterations, and loss of heterozygosity patterns. Endometrial glandular dysplasia is a focal lesion, involving single glands or small groups of glands, usually measuring <1 mm in size. Cells show hyperchromatic nuclei, but never reaching the degree of nuclear atypia seen in SC. Endometrial glandular dysplasia is more frequently seen in association with SC (53%) than with EEC (2%). Endometrial intraepithelial carcinoma (EIC) is characterized by replacement of the surface endometrial epithelium by highly atypical cells with extension to endometrial glands, with identical cytological features to invasive SC, but without stromal invasion. However, the diagnosis of EIC is discouraged, as it may be associated with high-stage disease and a fatal outcome. EIC cells may spread to the peritoneal surface via transtubal spread of tumor cells from the uterine cavity, even in absence of myometrial invasion. EIC may be found in isolation or, more commonly, adjacent to a focus of invasive SC.17, 18, 19, 20, 21 Asuggested terminology for such tumors includes ‘early/limited’ SC.14
Prognosis
Uterine SCs are aggressive tumors.3, 4, 5 Patients often have advanced stage tumors at initial presentation. First-line treatment is surgery. Platinum-based chemotherapy has been shown to be less effective in comparison with SC originating from fallopian tube/ovary. Surgical staging should include total hysterectomy with bilateral salpingo-oophorectomy, regional lymph node dissection, omentectomy, and peritoneal biopsies. The overall survival rate for all stages is ~30–40%.22, 23, 24, 25, 26, 27, 28 Patients with FIGO stage I disease have the highest 5-year survival rates.29 Prognosis for SC restricted to the endometrium, or exclusively involving an endometrial polyp is much better, reaching 80% survival at 5 years in some series. Size and extent of lymphovascular invasion are also prognostically important.30, 31, 32 The tumor may spread to cervix, fallopian tubes, ovaries, lymph nodes, peritoneum, and pleura.33 The microscopic appearance of endometrial SC metastasizing to the peritoneum is very similar to metastatic ovarian SC. WT-1 immunostaining is helpful in this differential diagnosis. However, it is worth mentioning that WT-1 is also expressed in a reduced number of uterine SC, but staining is usually weak to moderate, in contrast to the strong and diffuse staining of ovarian SC.
Differential diagnosis
High-Grade EEC
SC may exhibit a prominent solid pattern causing problems in differential diagnosis with high-grade EEC (Table 1). Identification of typical features of EEC or SC is helpful. It is important to pay attention to true glandular differentiation, and also squamous, mucinous, or secretory change, which favors EEC (Figure 8). Coexistence with complex endometrial hyperplasia/endometrioid intraepithelial neoplasia favors an EEC, whereas the presence of EIC would support the diagnosis of SC.34, 35, 36, 37 There are reports suggesting that high-grade EEC with mutations in POLE may be particularly prone to exhibit features mimicking SC,38, 39, 40 including predominant solid growth, marked pleomorphism.
Low-Grade EEC
This is a very important problem41 (Table 1), as there is a subset of SC that shows a prominent glandular pattern, simulating a low-grade EEC at low-power magnification.35, 36, 37 There is usually great discordance between the architectural and cytological features of the tumor. Tumor cells show high-grade atypical features with high nuclear-to-cytoplasmic ratio, prominent nuclear pleomorphism, enlarged hyperchromatic nuclei, and prominent nucleoli. Presence of obvious endometrioid differentiation, such as squamous, mucinous, or secretory change, goes against the diagnosis of SC. In contrast, SC shows marked pseudostratification, and lack of nuclear polarity (Figure 9), or true luminal border. It is important to pay attention to the myoinvasive front of the tumor. Patterns such as microcystic elongated fragmented favor EEC, whereas the typical "gaping gland" appearance is characteristic of SC.
Another important differential diagnosis of SC is low-grade EEC with papillary (villoglandular) pattern.42, 43 The latter shows elongated villous papillae, lacking the complex papillary pattern, as well as prominent cell stratification and papillary tufts, striking pleomorphism, and macronucleoli of SC.
Finally, SC may be confused with low-grade EEC with small nonvillous papillae (Figure 10).44 This variant accounts for 8% of EEC. The tumors contain small papillae within the endometrioid glands. The papillae are composed of buds of cells (not true papillae as in SC) with large eosinophilic cytoplasm, which typically show low-grade cytological features.
Mixed endometrioid–serous carcinoma and ambiguous tumors
SC usually occurs in pure form; however, occasionally, it may coexist with EEC.45, 46, 47 It has been suggested that the serous component may arise as a result of progression of the endometrioid elements. When the second of these components is present in at least 5% of the tumor, the designation of mixed endometrial carcinoma is used, being mixed endometrioid and SC (mixed EEC-SC) the most frequent combination. The correct diagnosis of the second component is crucial to determine treatment options and outcome for these patients,45, 46, 48 as it has been suggested that the presence of as little as 10% of a type-II component could adversely affect patient’s outcome. There is some interobserver variation in histological typing in endometrial carcinoma. This is partly due to the fact that some EEC may exhibit papillary arrangements and may be erroneously mistaken as SC. On the other side, some pure SC may show a glandular growth that may be misinterpreted as EEC. Inappropriate interpretation of these unusual patterns may lead to incorrect diagnosis of mixed EEC-SC. To avoid an incorrect diagnosis of either pure EEC or SC as mixed EEC-SC, rigorous criteria should be used, and diagnosis should be confirmed with the help of immunohistochemistry or molecular tools.
Mixed EEC-SCs are ambiguous tumors. The microdissected EEC component has molecular features of EEC but also some features of SC. On the other hand, the microdissected SC component has molecular characteristics of SC (p53 mutations), but retains EEC features (K-RAS and PTEN mutations). Furthermore, there is a small group of high-grade EC tumors that are molecular and morphologically ambiguous. Classification of this small subset of tumors into SC or EEC is difficult and probably artificial.47
Clear cell carcinoma
As mentioned earlier, SC may exhibit cells with clear cytoplasm. These tumors should not be misinterpreted as clear cell carcinoma or mixed serous–clear cell carcinoma. Rigorous interpretation of the clear cell component is recommended when analyzing a SC.49 Endometrial clear cell carcinoma is similar to its ovarian counterpart. Microscopically, they are characterized by a variety of patterns such as tubulocystic, papillary, glandular, and solid. Most clear cell carcinomas show mixed patterns. Tumor cells may exhibit a prominent clear appearance, due to abundant glycogen. Tumor cells may also show a hobnail configuration, which results from secretion of the cytoplasmic contents into the lumina, so the nuclei appear bulbous and protrude into the central aspects of the glands. Cystic spaces are common. Hyaline bodies are very frequent and characteristic. Clear cell adenocarcinoma usually contains small round papillae with hyalinized cores, and does not show the typical pseudostratification of SC. The nuclei are pleomorphic but uniform, with prominent nucleoli. In contrast to SC, mitotic index is low. The lumen of the glandular spaces usually contains mucin. Sheets of large cells with abundant eosinophilic (oncocytic or oxyphilic) cytoplasm, may be seen and may predominate occasionally. Clear cell carcinoma is usually (but not always) negative for estrogen receptor, and show a wild-type pattern for p53 staining. Alpha-methylacyl-CoA racemase (AMACR) and Napsin A are helpful in confirming this diagnosis.50, 51, 52 Staining for Napsin A was seen in 87% of clear cell carcinomas, whereas only in 8% SC and no EEC in one study.50 Moreover, AMACR immunoreactivity was seen in 75% of clear cell carcinomas, but only in 22% of EEC and 15% of SC.51 It has been shown that hepatocyte nuclear factor 1-beta immunoreactivity lacks specificity per the diagnosis of endometrial clear cell carcinoma.53
Carcinosarcoma
The differential diagnosis between carcinosarcoma (malignant mixed müllerian tumor) and SC may be very difficult in small biopsies and curettages, as SC elements may predominate as the epithelial component of carcinosarcoma. The recognition of a biphasic pattern, with an obvious typical sarcomatous component, is the most important criterion for the diagnosis.
Secondary uterine involvement of adnexal serous carcinoma
SCs originating from the fallopian tube/ovary may spread to the uterus. If the SC is high grade, the differential diagnosis may be very difficult. WT-1 positivity is much more common in SC from fallopian tube/ovary in comparison with those from the endometrium; in the latter, if positive, it typically shows focal and weak staining. Moreover, drop metastases from fallopian tube/ovarian SC tend to be multifocal with small, free floating nests of tumor cells, and not associated with endometrial polyps.54, 55 Abundant psammoma bodies slightly favor upper gynecologic tract primary origin.
Diagnosing SC in small biopsies
The diagnosis of SC may be difficult in small endometrial biopsies. It is important to look for typical features of SC, such as papillae, cell stratification and budding, slit-like spaces, and highly atypical cells. It is also important to look for lack of EEC features, such as squamous and mucinous areas, nuclear polarity, or luminal border. The presence of marked discordance between the low-grade architectural and the high-grade cytological features should alert one to the possibility of SC in a gland-forming EC. Rigorous interpretation of clear cell elements and appropriate assessment of tumor stroma may be helpful in the differential diagnosis with clear cell carcinoma or carcinosarcoma. Immunohistochemistry (p53, p16, ER, IMP2, IMP3, PTEN, and Arid1A) is in general very helpful, but individual cases may show discordant features. A diagnosis of ‘high-grade endometrial carcinoma, serous carcinoma component cannot be excluded’ is acceptable as a managerial approach in some instances. It is important to convey that the carcinoma is high grade and that a serous component cannot be excluded, so it could be taken into account at the time of final surgery.
Molecular features of SC
cDNA array studies have demonstrated that the expression profiling of EEC is different from that of SC. In one study, 191 genes exhibited a greater than twofold differences between 10 EECs and 16 SCs. One of the genes, FOLR TFF3 was significantly upregulated in SC.55 In another study, a different expression profile was seen between EEC and SC. Interestingly, estrogen-regulated genes were upregulated in EEC, whereas SC showed increased expression of genes involved in the regulation of the mitotic spindle checkpoint.56 Two additional studies demonstrated differentially expression between EECs and SC.57 The methylation profile of SC is also different from that of EEC; methylation of CDKN2B and TP73 was quite typical of SC.58
P53 is the most frequent mutated gene (90%), probably involved in early stages of tumor development, as it is frequently abnormal in SC restricted to the endometrial surface, without myometrial invasion. P53 mutations may be demonstrated by immunohistochemistry showing the typical ‘all or nothing’ pattern (strong diffuse positivity or total absence of staining), which contrasts with the ‘wild-type’ pattern, characterized by focal, weak, and somewhat variable staining from area to area (Figure 11).59 HER-2 has been shown to be amplified in SC in some series, but results have been inconsistently reproduced, whereas EGFR has been shown to be overexpressed in 36–56% of SC. PIK3CA is frequently mutated (15–41%) in these tumors.60
The Cancer Genome Atlas Research Network (TCGA) has recently performed an integrating genomic characterization of EC.61 Exome-sequence analysis revealed four groups of tumors. Group 1, comprised of EEC with mutations in POLE associated with good prognosis. Group 2, including EEC with microsatellite instability and group 3 tumors including EEC with low copy-number alterations, both showing similar progression-free survival rates, intermediate between groups 1 and 4. Group 4 (serous like) showed high number of gene copy-number alterations and p53 mutations, and worse prognosis, and was composed of most (but not all) SC, but also some EEC (many EEC3, but also some EEC1–2). In other words, there are tumors that are morphologically EEC but molecularly similar to SC, and also tumors that are microscopically SC, but molecularly similar to EEC. These ambiguous tumors show discordant microscopic/molecular features. The genes most frequently mutated in SC, according to TCGA are p53 (90.7%), PIK3CA (41.9%), FBXW/ (30.2%), and PPP2R1A (36.6%). Additional studies using exome-sequencing analysis have shown mutations in TAF-1 (30%). The TCGA prognostic classification has been recently validated in 152 endometrial carcinoma cases.62, 63
Utility of immunohistochemical and molecular tools in distinguishing SC from EEC
Histological type has consistently been proved to be an important predictor of survival, but also a determinant for the extent of the initial surgical procedure and subsequent use of adjuvant therapy. There is moderate to excellent agreement (0.62–0.87) in overall histologic typing, but also significant interobserver variability among high-grade tumors. In one study, 56 high-grade ECs were reviewed by three expert gynecological pathologists. Agreement between all three reviewers was obtained in 35 (62.5%) of cases, regarding the subtype diagnosis of the exclusive or predominant component. Major disagreement was noted in the remaining 20 cases (35.8%). The most frequent areas of disagreement were SC versus CC (seven cases) and SC versus EEC3 (six cases). Immunostaining for five biomarkers (p16, ER, PR, PTEN, and p53) improved histotype diagnosis in the most controversial cases.41 In a different study, eight experienced gynecological pathologists reviewed online slides before and after receiving p53, p16, and ER immunostaining results. The average κ-values was 0.55 on the basis of microscopical examination, but improved to 0.68 after considering immunohistochemical results.64
Several IHC markers have been shown to be differentially expressed in EEC, including EEC3, as opposed to SC (Table 2). Some of these markers were previously found to be differentially expressed between EEC and SC by cDNA analysis and also differentially abnormal by mutation analysis. For example, several studies have shown decreased expression of ER in SC, and also a lower frequency of PTEN alterations in SC in comparison with EEC. Additional proteins that are differentially expressed in EEC and SC are p16, HER2,65 claudin 3 and 4,66 Nrf2,67 p53,68, 69 p16,70 FOLR-1,71 HMGA-2,72, 73 cyclin E,74 IMP2,75 and IMP3 (ref. 76; Figure 12), although p16 expression may be quite strong and diffuse77 (Figure 13).
As mentioned before, HER2 amplification and overexpression has been found more frequently in SC in some but not all series.65 Claudin 3 and 4 are transmembrane tight junction proteins, frequently overexpressed in SC by immunohistochemistry, but subsequently confirmed by rt-PCR.66 Nrf2 is a significant nuclear transcription factor that maintains intracellular redox homeostasis through regulating the transcription of target genes, with an antioxidant role.67 HMGA-2 (high-mobility group AT-hook 2) is a non-histone DNA-binding factor that binds to AT-rich sequences in the minor groove of the DNA helix.72, 73 IMP2 and 3 are oncofetal mRNA-binding proteins, members of the human IGF-II mRNA-binding protein family. Folate receptor alpha (FOLR1) is a membrane-bound receptor, involved in the transport of folate, as well as other regulatory cellular processes.71
Several authors have attempted the use of panels of antibodies to help in diagnosis and prognosis of EC. Alkushi et al.78 examined the expression profile of 12 markers (bcl-2, ER, p53, p21, p27, HER-2, E2F1, p63, PTEN, Gfi-1, B72.3, and CK5/6) in 200 EC (including 156 EEC and 13 SC) using a TMA. Seven of the 12 markers (p53, ER, bcl-2, HER-2, p27, E2F1, and PTEN) showed prognostic significance in univariate analysis. The cluster group designation was performed based on eight markers (p53, ER, bcl-2, HER-2, p27, E2F1, PTEN, and p21), and correlated with tumor grade, stage and cell type. Reid Nicholson examined 126 EC (including 42 EEC1–2, 40 EEC3, and 24 SC) using five immunomarkers. Substantial immunophenotypic diversity was observed among EC.79 Only 70% of EEC 1 and 2 and 26% of EEC3 exhibited the typical phenotype of EEC (p16-, ER+, PR+, mCEA-, and vimentin+). Alkushi80 also assessed the usefulness of a panel of six antibodies (ER, IMP3, p16, p53, PR, and PTEN), in a series of 180 EC including a subgroup of 58 high-grade carcinomas (34 EEC3, and 15 SC). They found that p16, PTEN, and IMP3 were statistically significantly more frequently expressed in SC in comparison with EEC3, whereas ER and p53 approached but did not reach significance. A combination of p16 and PTEN predicted EEC3 versus SC with a sensitivity of 90.0% and specificity of 96.8%. Han et al.81 assessed the interobserver agreement on histological type in high-grade EC, by morphology, but also by using immunomarker combinations with six routine (p53, p16, ER, PR, Ki-67, and vimentin) and six experimental markers (PTEN, ARID1A, CTNNB1, IMP3, HNF1B, and TFF3). Consensus about histological type based on morphology reached 72%, but increased to 100% when markers were used, and a three-panel marker composed of p53, ER, and p16, was recommended to help in the distinction between EEC and SC. Wei82 reviewed 358 biopsies of EC, and assessed the usefulness of immunohistochemistry (ER, PR, p53, p16, vimentin, and in some selected cases also beta-catenin, WT-1, HER-2, HMGA-2, and Ki-67) in 41 cases with differing interpretation (EEC versus NEEC) between the referring pathologist and second reviewer. Of the 41 cases, 10 were classified as EEC, 18 as SC, and diagnosis remained indeterminate in 5 cases. Recently, McConechy et al.83 performed target-enrichment sequencing on 393 EC, by sequencing nine genes (ARID1A, PPP2R1A, PTEN, PIK3CA, KRAS, CTNNB1, P53, BRAF, and PPP2R5C), and suggest that the nine-gene panel may be useful as an adjunct to morphological classification of EC. In a recent study, we have used IHC for several proteins, previously shown to be differentially expressed in EEC and SC by cDNA and protein analysis. As a result, we identified nine conditions that allowed prediction of EEC (IMP3⩾2, IMP2⩾115, p53⩾20, HMGA2⩾30, FolR1⩾50, p16⩾170, CycE1⩾220, nuclear PTEN⩾2, and ER⩽50). The performance of this signature was remarkably solid, with good interobserver agreement.84
Bullet practical points
-
SC is an aggressive type of endometrial carcinoma that does not infrequently poses problems in differential diagnosis, especially (but not limited) in finding pseudoglandular and solid patterns or clear cells.
-
The most helpful features for the diagnosis of serous carcinoma are as follows: (1) architectural and cytological discordance, ragged luminal borders, slit-like spaces, cell stratification with lack of polarity±budding, highly atypical cells, as well as lack of endometrioid features; (2) conventional areas if solid; and (3) absence of typical architectural patterns of clear cell carcinoma if clear cells.
-
If in biopsy/curettage, the diagnosis of serous carcinoma cannot be made with confidence, it is still important to convey that the carcinoma is high grade and that a serous component cannot be excluded, so it could be taken into account at the time of final surgery.
-
Immunohistochemistry particularly p53, p16, IMP2, IMP3, and molecular studies may be helpful adjuncts but one should correlate with morphology as some degree of overlap occur.
References
Clement PB, Young RH . Non-endometrioid carcinomas of the uterine corpus: a review of their pathology with emphasis on recent advances and problematic aspects. Adv Anat Pathol 2004;11:117–142.
Matthews RP, Hutchinson-Colas J, Maiman M et al. Papillary serous and clear cell type lead to poor prognosis of endometrial carcinoma in black women. Gynecol Oncol 1997;65:206–212.
Ng JS, Han AC, Edelson MI et al. Uterine papillary serous carcinoma presenting as distant lymph node metastasis. Gynecol Oncol 2001;80:417–420.
del Carmen MG, Birrer M, Schorge JO . Uterine papillary serous cancer: a review of the literature. Gynecol Oncol 2012;127:651–661.
Hamilton CA, Cheung MK, Osann K et al. Uterine papillary serous and clear cell carcinomas predict for poorer survival compared to grade 3 endometrioid corpus cancers. Br J Cancer 2006;94:642–646.
Kurman RJ, Carcangiu ML, Herrington CS et alWHO classification of tumors of the female reproductive organs. IARC press: Lyon, France, 2014.
Hendrickson M, Ross J, Eifel P et al. Uterine papillary serous carcinoma: a highly malignant form of endometrial adenocarcinoma. Am J Surg Pathol 1982;6:93–108.
Factor SM . Papillary adenocarcinoma of the endometrium with psammoma bodies. Arch Pathol 1974;98:201–205.
Lauchlan SC . Tubal (serous) carcinoma of the endometrium. Arch Pathol Lab Med 1981;105:615–618.
Sherman ME, Bitterman P, Rosenshein NB et al. Uterine serous carcinoma. A morphologically diverse neoplasm with unifying clinicopathologic features. Am J Surg Pathol 1992;16:600–610.
Carcangiu ML, Tan LK, Chambers JT et al. Uterine serous carcinoma: a study of 13 cases. Am J Surg Pathol 1997;21:1507–1514.
Prat J, Oliva E, Lerma E et al. Uterine papillary serous adenocarcinoma. A 10-case study of p53 and c-erbB-2 expression and DNA content. Cancer 1994;74:1778–1783.
Faratian D, Stillie A, Busby-Earle RM et al. A review of the pathology and management of uterine papillary serous carcinoma and correlation with outcome. Int J Gynecol Cancer 2006;16:972–978.
Lim D, Oliva E . Nonendometrioid endometrial carcinomas. Semin Diagn Pathol 2010;27:241–260.
Zheng W, Liang SX, Yu H et al. Endometrial glandular dysplasia: a newly defined precursor lesion of uterine papillary serous carcinoma. Part I: morphologic features. Int J Surg Pathol 2004;12:207–223.
Jarboe EA, Pizer ES, Miron A et al. Evidence for a latent precursor (p53 signature) that may precede serous endometrial intraepithelial carcinoma. Mod Pathol 2009;22:345–350.
Dotto J, Tavassoli FA . Serous intraepithelial carcinoma arising in an endometrial polyp: a proposal for modification of terminology. Int J Surg Pathol 2008;16:8–10.
McCluggage WG, Sumathi VP, McManus DT . Uterine serous carcinoma and endometrial intraepithelial carcinoma arising in endometrial polyps: report of 5 cases, including 2 associated with tamoxifen therapy. Hum Pathol 2003;34:939–943.
Silva EG, Jenkins R . Serous carcinoma in endometrial polyps. Mod Pathol 1990;3:120–128.
Wheeler DT, Bell KA, Kurman RJ et al. Minimal uterine serous carcinoma: diagnosis and clinicopathologic correlation. Am J Surg Pathol 2000;24:797–806.
Ambros RA, Sherman ME, Zahn CM et al. Endometrial intraepithelial carcinoma: a distinctive lesion specifically associated with tumors displaying serous differentiation. Hum Pathol 1995;26:1260–1267.
Chambers JT, Merino M, Kohorn EI et al. Uterine papillary serous carcinoma. Obstet Gynecol 1987;69:109–113.
Cirisano FD Jr, Robboy SJ, Dodge RK et al. Epidemiologic and surgicopathologic findings of papillary serous and clear cell endometrial cancers when compared to endometrioid carcinoma. Gynecol Oncol 1999;74:385–394.
Lee KR, Belinson JL . Recurrence in noninvasive endometrial carcinoma. Relationship to uterine papillary serous carcinoma. Am J Surg Pathol 1991;15:965–973.
Carcangiu ML, Chambers JT . Uterine papillary serous carcinoma: a study on 108 cases with emphasis on the prognostic significance of associated endometrioid carcinoma, absence of invasion, and concomitant ovarian carcinoma. Gynecol Oncol 1992;47:298–305.
Rosenberg P, Boeryd B, Simonsen E . A new aggressive treatment approach to high-grade endometrial cancer of possible benefit to patients with stage I uterine papillary cancer. Gynecol Oncol 1993;48:32–37.
Carcangiu ML, Chambers JT . Early pathologic stage clear cell carcinoma and uterine papillary serous carcinoma of the endometrium: comparison of clinicopathologic features and survival. Int J Gynecol Pathol 1995;14:30–38.
Soslow RA, Bissonnette JP, Wilton A et al. Clinicopathologic analysis of 187 high-grade endometrial carcinomas of different histologic subtypes: similar outcomes belie distinctive biologic differences. Am J Surg Pathol 2007;31:979–987.
Abeler VM, Kjorstad KE . Serous papillary carcinoma of the endometrium: a histopathological study of 22 cases. Gynecol Oncol 1990;39:266–271.
Winer I, Ahmed QF, Mert I et al. Significance of lymphovascular space invasion in uterine serous carcinoma: what matters more; extent or presence? Int J Gynecol Pathol 2015;34:47–56.
Semaan A, Mert I, Munkarah AR et al. Clinical and pathologic characteristics of serous carcinoma confined to the endometrium: a multi-institutional study. Int J Gynecol Pathol 2013;32:181–187.
Winer I, Mahdi H, Bandyopadhyay S et al. Correlation of tumor size with other prognostic factors in uterine serous carcinoma: a large multi-institutional study. Gynecol Oncol 2013;128:316–321.
Goff BA, Kato D, Schmidt RA et al. Uterine papillary serous carcinoma: patterns of metastatic spread. Gynecol Oncol 1994;54:264–268.
Darvishian F, Hummer AJ, Thaler HT et al. Serous endometrial cancers that mimic endometrioid adenocarcinomas: a clinicopathologic and immunohistochemical study of a group of problematic cases. Am J Surg Pathol 2004;28:1568–1578.
Bartosch C, Manuel Lopes J, Oliva E . Endometrial carcinomas: a review emphasizing overlapping and distinctive morphological and immunohistochemical features. Adv Anat Pathol 2011;18:415–437.
Garg K, Soslow RA . Strategies for distinguishing low-grade endometrioid and serous carcinomas of endometrium. Adv Anat Pathol 2012;19:1–10.
Soslow RA . High-grade endometrial carcinomas - strategies for typing. Histopathology 2013;62:89–110.
Meng B, Hoang LN, McIntyre JB et al. POLE exonuclease domain mutation predicts long progression-free survival in grade 3 endometrioid carcinoma of the endometrium. Gynecol Oncol 2014;134:15–19.
Church DN, Stelloo E, Nout RA et al. Prognostic Significance of POLE Proofreading Mutations in Endometrial Cancer. J Natl Cancer Inst 2014;107:402.
Hussein YR, Weigelt B, Levine DA et al. Clinicopathological analysis of endometrial carcinomas harboring somatic POLE exonuclease domain mutations. Mod Pathol 2015;28:505–514.
Gilks CB, Oliva E, Soslow RA . Poor interobserver reproducibility in the diagnosis of high-grade endometrial carcinoma. Am J Surg Pathol 2013;37:874–881.
Ambros RA, Malfetano JH . Villoglandular adenocarcinoma of the endometrium. Am J Surg Pathol 1998;22:1379–1385.
Ambros RA, Ballouk F, Malfetano JH et al. Significance of papillary (villoglandular) differentiation in endometrioid carcinoma of the uterus. Am J Surg Pathol 1994;18:569–575.
Murray SK, Young RH, Scully RE . Uterine endometrioid carcinoma with small nonvillous papillae: an analysis of 26 cases of a favourable-prognosis tumor to be distinguished from serous carcinoma. Int J Surg Pathol 2000;8:279–289.
Quddus MR, Sung CJ, Zhang C et al. Minor serous and clear cell components adversely affect prognosis in ''mixed-type'' endometrial carcinomas: a clinicopathologic study of 36 stage-I cases. Reprod Sci 2010;17:673–678.
Roelofsen T, van Ham MA, Wiersma van Tilburg JM et al. Pure compared with mixed serous endometrial carcinoma: two different entities? Obstet Gynecol 2012;120:1371–1381.
Soslow RA . Endometrial carcinomas with ambiguous features. Semin Diagn Pathol 2010;27:261–273.
Williams KE, Waters ED, Woolas RP et al. Mixed serous-endometrioid carcinoma of the uterus: pathologic and cytopathologic analysis of a high-risk endometrial carcinoma. Int J Gynecol Cancer 1994;4:7–18.
Fadare O, Zheng W, Crispens MA et al. Morphologic and other clinicopathologic features of endometrial clear cell carcinoma: a comprehensive analysis of 50 rigorously classified cases. Am J Cancer Res 2013;3:70–95.
Fadare O, Desouki MM, Gwin K et al. Frequent expression of napsin A in clear cell carcinoma of the endometrium: potential diagnostic utility. Am J Surg Pathol 2014;38:189–196.
Fadare O, Parkash V, Gwin K et al. Utility of α-methylacyl-coenzyme-A racemase (p504s) immunohistochemistry in distinguishing endometrial clear cell carcinomas from serous and endometrioid carcinomas. Hum Pathol 2013;44:2814–2821.
Lim D, Ip PP, Cheung AN et al. Immunohistochemical comparison of ovarian and uterine endometrioid carcinoma, endometrioid carcinoma with clear cell change, and clear cell carcinoma. Am J Surg Pathol 2015;39:1061–1069.
Fadare O, Liang SX . Diagnostic utility of hepatocyte nuclear factor 1-beta immunoreactivity in endometrial carcinomas: lack of specificity for endometrial clear cell carcinoma. Appl Immunohistochem Mol Morphol 2012;20:580–587.
Euscher ED1, Malpica A, Deavers MT et al. Differential expression of WT-1 in serous carcinomas in the peritoneum with or without associated serous carcinoma in endometrial polyps. Am J Surg Pathol 2005;29:1074–1078.
Jarboe EA, Miron A, Carlson JW et al. Coexisting intraepithelial serous carcinomas of the endometrium and fallopian tube: frequency and potential significance. Int J Gynecol Pathol 2009;28:308–315.
Moreno-Bueno G, Sánchez-Estévez C, Cassia R . Differential gene expression profile in endometrioid and nonendometrioid endometrial carcinoma: STK15 is frequently overexpressed and amplified in nonendometrioid carcinomas. Cancer Res 2003;63:5697–5702.
Cao QJ, Belbin T, Socci N et al. Distinctive gene expression profiles by cDNA microarrays in endometrioid and serous carcinomas of the endometrium. Int J Gynecol Pathol 2004;23:321–329.
Seeber LM, Zweemer RP, Marchionni L et al. Methylation profiles of endometrioid and serous endometrial cancers. Endocr Relat Cancer 2010;17:663–673.
McCluggage WG, Soslow RA, Gilks CB . Patterns of p53 immunoreactivity in endometrial carcinomas: 'all or nothing' staining is of importance. Histopathology 2011;59:786–788.
Hayes MP, Ellenson LH . Molecular alterations in uterine serous carcinoma. Gynecol Oncol 2010;116:286–289.
Cancer Genome Atlas Research Network Integrated genomic characterization of endometrial carcinoma. Integrated genomic characterization of endometrial carcinoma. Nature 2013;497:67–73.
Talhouk A, McConechy MK, Leung S et al. A clinically applicable molecular-based classification for endometrial cancers. Br J Cancer 2015;113:299–310.
Stelloo E, Bosse T, Nout RA et al. Refining prognosis and identifying targetable pathways for high-risk endometrial cancer; a TransPORTEC initiative. Mod Pathol 2015;28:836–844.
Hoang LN, McConechy MK, Köbel M et al. Histotype-genotype correlation in 36 high-grade endometrial carcinomas. Am J Surg Pathol 2013;37:1421–1432.
Morrison C, Zanagnolo V, Ramirez N et al. HER-2 is an independent prognostic factor in endometrial cancer: association with outcome in a large cohort of surgically staged patients. J Clin Oncol 2006;24:2376–2385.
Konecny GE, Agarwal R, Keeney GA et al. Claudin-3 and claudin-4 expression in serous papillary, clear-cell, and endometrioid endometrial cancer. Gynecol Oncol 2008;109:263–269.
Chen N, Yi X, Abushahin N et al. Nrf2 expression in endometrial serous carcinomas and its precancers. Int J Clin Exp Pathol 2010;4:85–96.
Alkushi A, Lim P, Coldman A et al. Interpretation of p53 immunoreactivity in endometrial carcinoma: establishing a clinically relevant cut-off level. Int J Gynecol Pathol 2004;23:129–137.
McCluggage WG, Soslow RA, Gilks CB . Patterns of p53 immunoreactivity in endometrial carcinomas:‘all or nothing’staining is of importance. Histopathology 2011;59:786–788.
Yemelyanova A, Ji H, Shih I et al. Utility of p16 expression for distinction of uterine serous carcinomas from endometrial endometrioid and endocervical adenocarcinomas: immunohistochemical analysis of 201 cases. Am J Surg Pathol 2009;33:1504–1514.
Dainty LA, Risinger JI, Morrison C et al. Overexpression of folate binding protein and mesothelin are associated with uterine serous carcinoma. Gynecol Oncol 2007;105:563–570.
McCluggage WG, Connolly LE, McBride HA et al. HMGA2 is commonly expressed in uterine serous carcinomas and is a useful adjunct to diagnosis. Histopathology 2012;60:547–553.
Romero-Pérez L, Castilla MÁ, López-García MÁ et al. Molecular events in endometrial carcinosarcomas and the role of high mobility group AT-hook 2 in endometrial carcinogenesis. Hum Pathol 2013;44:244–254.
Cassia R, Moreno‐Bueno G, Rodríguez‐Perales S et al. Cyclin E gene (CCNE) amplification and hCDC4 mutations in endometrial carcinoma. J Pathol 2013;201:589–595.
Zhang L, Liu Y, Hao S et al. IMP2 expression distinguishes endometrioid from serous endometrial adenocarcinomas. Am J Surg Pathol 2011;35:868–872.
Zheng W, Yi X, Fadare O et al. The oncofetal protein IMP3: a novel biomarker for endometrial serous carcinoma. Am J Surg Pathol 2008;32:304–315.
Alvarez T, Miller E, Duska L et al. Molecular profile of grade 3 endometrioid endometrial carcinoma: is it a type I or type II endometrial carcinoma? Am J Surg Pathol 2012;36:753–761.
Alkushi A, Clarke BA, Akbari M et al. Identification of prognostically relevant and reproducible subsets of endometrial adenocarcinoma based on clustering analysis of immunostaining data. Mod Pathol 2007;20:1156–1165.
Reid-Nicholson M, Iyengar P, Hummer AJ et al. Immunophenotypic diversity of endometrial adenocarcinomas: implications for differential diagnosis. Mod Pathol 2006;19:1091–1100.
Alkushi A, Kobel M, Kalloger SE et al. High-grade endometrial carcinoma: serous and grade 3 endometrioid carcinomas have different immunophenotypes and outcomes. Int J Gynecol Pathol 2010;29:343–350.
Han G, Sidhu D, Duggan MA et al. Reproducibility of histological cell type in high-grade endometrial carcinoma. Mod Pathol 2013;26:1594–1604.
Wei JJ, Paintal A, Keh P . Histologic and immunohistochemical analyses of endometrial carcinomas: experiences from endometrial biopsies in 358 consultation cases. Arch Pathol Lab Med 2013;137:1574–1583.
McConechy MK, Ding J, Cheang MC et al. Use of mutation profiles to refine the classification of endometrial carcinomas. J Pathol 2012;228:20–30.
Santacana M, Maiques O, Valls J et al. A 9-protein biomarker molecular signature for predicting histologic type in endometrial carcinoma by immunohistochemistry. Hum Pathol 2014;45:2394–2403.
Acknowledgements
The study was supported by grants, ISCIII PI13/01701, Fundacio La Marató TV3 (2C/C2013), 2014SGR138, RD12/0036/0013, Fundación Científica Asociación Española contra el Cancer.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Gatius, S., Matias-Guiu, X. Practical issues in the diagnosis of serous carcinoma of the endometrium. Mod Pathol 29 (Suppl 1), S45–S58 (2016). https://doi.org/10.1038/modpathol.2015.141
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/modpathol.2015.141
This article is cited by
-
Updated endometrial cancer FIGO staging: the role of MRI in determining newly included histopathological criteria
Abdominal Radiology (2024)
-
Are the uterine serous carcinomas underdiagnosed? Histomorphologic and immunohistochemical correlates and clinical follow up in high-grade endometrial carcinomas initially diagnosed as high-grade endometrioid carcinoma
Modern Pathology (2018)
-
The evolution of endometrial carcinoma classification through application of immunohistochemistry and molecular diagnostics: past, present and future
Virchows Archiv (2018)