Abstract
Efficient boosting of memory T-cell numbers to protective levels generally requires a relatively long interval between immunizations. Decreasing this interval could be crucial in biodefense and cancer immunotherapy, in which rapid protective responses are essential. Here, we show that vaccination with peptide-coated dendritic cells (DCs) generated CD8+ T cells with the phenotype and function of memory cells within 4–6 d. These early memory CD8+ T cells underwent vigorous secondary expansion in response to a variety of booster immunizations, leading to elevated numbers of effector and memory T cells and enhanced protective immunity. Coinjection of CpG oligodeoxynucleotides, potent inducers of inflammation that did not alter the duration of DC antigen display, prevented the rapid generation of memory T cells in wild-type mice but not in mice lacking the interferon (IFN)-γ receptor. These data show that DC vaccination stimulates a pathway of accelerated generation of memory T cells, and suggest that events of inflammation, including the action of IFN-γ on the responding T cells, control the rate of development of memory CD8+ T cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Kaech, S.M., Wherry, E.J. & Ahmed, R. Effector and memory T-cell differentiation: implications for vaccine development. Nat. Rev. Immunol. 2, 251–262 (2002).
Badovinac, V.P., Porter, B.B. & Harty, J.T. Programmed contraction of CD8(+) T-cells after infection. Nat. Immunol. 3, 619–626 (2002).
Badovinac, V.P., Messingham, K.A., Hamilton, S.E. & Harty, J.T. Regulation of CD8+ T-cells undergoing primary and secondary responses to infection in the same host. J. Immunol. 170, 4933–4942 (2003).
Woodland, D.L. Jump-starting the immune system: prime–boosting comes of age. Trends Immunol. 25, 98–104 (2004).
Mercado, R. et al. Early programming of T-cell populations responding to bacterial infection. J. Immunol. 165, 6833–6839 (2000).
Kaech, S.M. & Ahmed, R. Memory CD8+ T-cell differentiation: initial antigen encounter triggers a developmental program in naive cells. Nat. Immunol. 2, 415–422 (2001).
van Stipdonk, M.J., Lemmens, E.E. & Schoenberger, S.P. Naive CTLs require a single brief period of antigenic stimulation for clonal expansion and differentiation. Nat. Immunol. 2, 423–429 (2001).
Wong, P. & Pamer, E.G. Cutting edge: antigen-independent CD8 T-cell proliferation. J. Immunol. 166, 5864–5868 (2001).
Kaech, S.M., Hemby, S., Kersh, E. & Ahmed, R. Molecular and functional profiling of memory CD8 T-cell differentiation. Cell 111, 837–851 (2002).
Sallusto, F., Geginat, J. & Lanzavecchia, A. Central Memory and Effector Memory T-cell Subsets: Function, Generation, and Maintenance. Annu. Rev. Immunol. 22, 683–709 (2004).
Wherry, E.J. et al. Lineage relationship and protective immunity of memory CD8 T-cell subsets. Nat. Immunol. 4, 225–234 (2003).
Roberts, A.D. & Woodland, D.L. Cutting Edge: Effector Memory CD8+ T-cells Play a Prominent Role in Recall Responses to Secondary Viral Infection in the Lung. J. Immunol. 172, 6533–6537 (2004).
Badovinac, V.P. & Harty, J.T. Memory lanes. Nat. Immunol. 4, 212–213 (2003).
Hamilton, S.E. & Harty, J.T. Quantitation of CD8+ T-cell expansion, memory, and protective immunity after immunization with peptide coated dendritic cells. J. Immunol. 169, 4936–4944 (2002).
Hamilton, S.E., Porter, B.B., Messingham, K.A.N., Badovinac, V.P. & Harty, J.T. MHC class Ia-restricted memory T-cells inhibit expansion of a nonprotective MHC class Ib (H2–M3)-restricted memory response. Nat. Immunol. 5, 159–168 (2004).
Pamer, E.G. Immune responses to Listeria monocytogenes . Nat. Rev. Immunol. 4, 812–823 (2004).
Wong, P., Lara-Tejero, M., Ploss, A., Leiner, I. & Pamer, E.G. Rapid development of T-cell memory. J. Immunol. 172, 7239–7245 (2004).
Kaech, S.M. et al. Selective expression of the interleukin 7 receptor identifies effector CD8 T-cells that give rise to long-lived memory cells. Nat. Immunol. 4, 1191–1198 (2003).
Schluns, K.S., Kieper, W.C., Jameson, S.C. & Lefrancois, L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T-cells in vivo. Nat. Immunol. 1, 426–432 (2000).
Schluns, K.S. & Lefrancois, L. Cytokine control of memory T-cell development and survival. Nat. Rev. Immunol. 3, 269–279 (2003).
Harrington, L.E., Galvan, M., Baum, L.G., Altman, J.D. & Ahmed, R. Differentiating between memory and effector CD8 T-cells by altered expression of cell surface O-glycans. J. Exp. Med. 191, 1241–1246 (2000).
An, L.L., Pamer, E. & Whitton, J.L. A recombinant minigene vaccine containing a nonameric cytotoxic-T-lymphocyte epitope confers limited protection against Listeria monocytogenes infection. Infect. Immun. 64, 1685–1693 (1996).
Brundage, R.A., Smith, G.A., Camilli, A., Theriot, J.A. & Portnoy, D.A. Expression and phosphorylation of the Listeria monocytogenes ActA protein in mammalian cells. Proc. Natl Acad. Sci. USA 90, 11890–11894 (1993).
Mackaness, G.B. Cellular resistance to infection. J. Exp. Med. 1, 381–406 (1962).
Edelson, B.T., Cossart, P. & Unanue, E.R. Cutting edge: paradigm revisited: antibody provides resistance to Listeria infection. J. Immunol. 163, 4087–4090 (1999).
Kerksiek, K.M., Busch, D.H., Pilip, I.M., Allen, S.E. & Pamer, E.G. H2–M3restricted T-cells in bacterial infection: rapid primary but diminished memory responses. J. Exp. Med. 190, 195–204 (1999).
Sprent, J. & Surh, C.D. T-cell memory. Annu. Rev. Immunol. 20, 551–579 (2002).
Seder, R.A. & Ahmed, R. Similarities and differences in CD4+ and CD8+ effector and memory T-cell generation. Nat. Immunol. 4, 835–842 (2003).
Hendriks, J., Gravestein, L.A., Tesselaar, K., van Lier, R.A., Schumacher, T.N. & Borst, J. CD27 is required for generation and long-term maintenance of T-cell immunity. Nat. Immunol. 1, 433–440.
Huster, K.M. et al. Selective expression of IL-7 receptor on memory T-cells identifies early CD40L-dependent generation of distinct CD8+ memory T-cell subsets. Proc. Natl Acad. Sci. USA 101, 5610–5615 (2004).
Badovinac, V.P., Porter, B.B. & Harty, J.T. CD8+ T-cell contraction is controlled by early inflammation. Nat. Immunol. 5, 809–817 (2004).
Shen, H. et al. Compartmentalization of bacterial antigens: differential effects on priming of CD8 T-cells and protective immunity. Cell 92, 535–545 (1998).
Hogquist, K.A. et al. T-cell receptor antagonist peptides induce positive selection. Cell 76, 17–27 (1994).
Foulds, K.E. et al. Cutting edge: CD4 and CD8 T-cells are intrinsically different in their proliferative responses. J. Immunol. 168, 1528–1532 (2002).
Banchereau, J. et al. Immunobiology of dendritic cells. Annu. Rev. Immunol. 18, 767–811 (2000).
Heath, W.R. & Carbone, F.R. Cross-presentation, dendritic cells, tolerance and immunity. Annu. Rev. Immunol. 19, 47–64 (2001).
Steinman, R.M., Hawiger, D. & Nussenzweig, M.C. Tolerogenic dendritic cells. Annu. Rev. Immunol. 21, 685–711 (2003).
Bouwer, H.G., Moors, M. & Hinrichs, D.J. Elimination of the listeriolysin O directed immune response by conservative alteration of the immunodominant listeriolysin O amino acid 91 to 99 epitope. Infect. Immun. 64, 3728–3735 (1996).
Krieg, A.M. CpG motifs: the active ingredient in bacterial extracts? Nat. Med. 9, 831–835 (2003).
Takeda, K., Kaisho, T. & Akira, S. Toll-like receptors. Annu. Rev. Immunol. 21, 335–376 (2003).
Wagner, H. Interactions between bacterial CpG-DNA and TLR9 bridge innate and adaptive immunity. Curr. Opin. Microbiol. 5, 62–69 (2002).
Haring, J.S., Corbin, G.A. & Harty, J.T. Dynamic regulation of IFN-γ signaling in antigen-specific CD8+ T-cells responding to infection. J. Immunol. 174, 6791–6802 (2005).
Badovinac, V.P., Tvinnereim, A.R. & Harty, J.T. Regulation of antigen specific CD8(+) T-cell homeostasis by perforin and interferon-gamma. Science 290, 1354–1357 (2000).
Paczesny, S. et al. Expansion of Melanoma-specific Cytolytic CD8+ T-cell Precursors in Patients with Metastatic Melanoma Vaccinated with CD34+ Progenitor-derived Dendritic Cells. J. Exp. Med. 199, 1503–1511 (2004).
Figdor, C.G., de Vries, I.J., Lesterhuis, W.J. & Melief, C.J.M. Dendritic cell immunotherapy: mapping the way. Nat. Med. 10, 475–480 (2004).
Pernis, A. et al. Lack of interferon γ receptor beta chain and the prevention of interferon γ signaling in TH1 cells. Science 269, 245–247 (1995).
Harty, J.T. & Bevan, M.J. Specific immunity to Listeria monocytogenes in the absence of IFN gamma. Immunity 3, 109–117 (1995).
Busch, D.H., Pilip, I.M., Vijh, S. & Pamer, E.G. Coordinate regulation of complex T-cell populations responding to bacterial infection. Immunity 8, 353–362 (1998).
Badovinac, V.P. & Harty, J.T. Intracellular staining for TNF and IFNgamma detects different frequencies of antigen-specific CD8(+) T-cells. J. Immunol. Methods 238, 107–117 (2000).
Badovinac, V.P., Hamilton, S.E. & Harty, J.T. Viral infection results in massive CD8+ T-cell expansion and mortality in vaccinated perforin deficient mice. Immunity 18, 463–474 (2003).
Acknowledgements
We thank R. Podyminogin for technical assistance, S. Perlman and S. Varga for critical review of the manuscript, J. L. Whitton for Vaccinia virus, L. Lefrancois and H. Shen for LM-OVA, H.G. Archie Bower for DP-L2528 and the US National Institutes of Health (NIH) tetramer core (Atlanta, Georgia) for providing tetramers. Supported by NIH grants AI42767, AI46653, AI50073 (to J.T.H.), T32AI0726 (to K.A.N.M and J.S.H) and T32AI007511 (to K.A.N.M).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
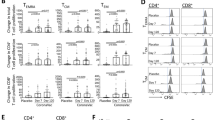
Supplementary Fig. 1
Increased frequencies of memory CD8+ T cells in lymphoid and nonlymphoid tissues in DC+LM mice. (PDF 168 kb)
Supplementary Fig. 2
The magnitude of expansion and memory CD8+ T-cell numbers in DC-immunized mice are determined by dose of L. monocytogenes booster infection. (PDF 95 kb)
Supplementary Fig. 3
Increased MHC class Ib CD8+ T-cell response after early booster infection of DC-fMIGWII immunized mice. (PDF 67 kb)
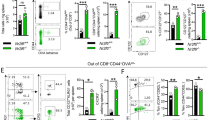
Supplementary Fig. 4
Antigen-specific CD8+ T cells exhibit phenotypic and functional characteristics of memory cells early after DC-peptide immunization. (PDF 69 kb)
Supplementary Fig. 5
DC-peptide immunization accelerates the transition of CD8+ T cells from an effector to memory phenotype. (PDF 71 kb)
Supplementary Fig. 6
CD8+ T-cell priming in the context of infection prevents accelerated memory generation and early prime-boost response. (PDF 69 kb)
Supplementary Fig. 7
In vitro maturation of DC with CpG does not prevent rapid memory CD8+ T-cell generation in vivo. (PDF 111 kb)
Rights and permissions
About this article
Cite this article
Badovinac, V., Messingham, K., Jabbari, A. et al. Accelerated CD8+ T-cell memory and prime-boost response after dendritic-cell vaccination. Nat Med 11, 748–756 (2005). https://doi.org/10.1038/nm1257
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/nm1257
This article is cited by
-
Universal immunotherapeutic strategy for hepatocellular carcinoma with exosome vaccines that engage adaptive and innate immune responses
Journal of Hematology & Oncology (2022)
-
Activated-memory T cells influence naïve T cell fate: a noncytotoxic function of human CD8 T cells
Communications Biology (2022)
-
Novel HLA-B7-restricted human metapneumovirus epitopes enhance viral clearance in mice and are recognized by human CD8+ T cells
Scientific Reports (2021)
-
Enhanced generation of influenza-specific tissue resident memory CD8 T cells in NK-depleted mice
Scientific Reports (2021)
-
Long-‘Trm’ protection of the CNS
Nature Immunology (2020)