Abstract
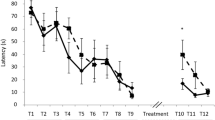
Retrieval of drug-associated memories is critical for maintaining addictive behaviors, as presentation of drug-associated cues can elicit drug seeking and relapse. Recently, we and others have demonstrated that β-adrenergic receptor (β-AR) activation is necessary for retrieval using both rat and human memory models. Importantly, blocking retrieval with β-AR antagonists persistently impairs retrieval and provides protection against subsequent reinstatement. However, the neural locus at which β-ARs are required for maintaining retrieval and subsequent reinstatement is unclear. Here, we investigated the necessity of dorsal hippocampus (dHipp) β-ARs for drug-associated memory retrieval. Using a cocaine conditioned place preference (CPP) model, we demonstrate that local dHipp β-AR blockade before a CPP test prevents CPP expression shortly and long after treatment, indicating that dHipp β-AR blockade induces a memory retrieval disruption. Furthermore, this retrieval disruption provides long-lasting protection against cocaine-induced reinstatement. The effects of β-AR blockade were dependent on memory reactivation and were not attributable to reconsolidation disruption as blockade of β-ARs immediately after a CPP test had little effect on subsequent CPP expression. Thus, cocaine-associated memory retrieval is mediated by β-AR activity within the dHipp, and disruption of this activity could prevent cue-induced drug seeking and relapse long after treatment.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Aston-Jones G, Rajkowski J, Kubiak P (1997). Conditioned responses of monkey locus coeruleus neurons anticipate acquisition of discriminative behavior in a vigilance task. Neuroscience 80: 697–715.
Bernardi RE, Ryabinin AE, Berger SP, Lattal KM (2009). Post-retrieval disruption of a cocaine conditioned place preference by systemic and intrabasolateral amygdala beta2- and alpha1-adrenergic antagonists. Learn Mem 16: 777–789.
Carroll KM, Nich C, Ball SA, McCance E, Rounsavile BJ (1998). Treatment of cocaine and alcohol dependence with psychotherapy and disulfiram. Addiction 93: 713–727.
Cassens G, Roffman M, Kuruc A, Orsulak PJ, Schildkraut JJ (1980). Alterations in brain norepinephrine metabolism induced by environmental stimuli previously paired with inescapable shock. Science 209: 1138–1140.
Childress AR, McLellan AT, O'Brien CP (1986). Role of conditioning factors in the development of drug dependence. Psychiatr Clin North Am 9: 413–425.
Corcoran KA, Maren S (2001). Hippocampal inactivation disrupts contextual retrieval of fear memory after extinction. J Neurosci 21: 1720–1726.
Debiec J, LeDoux JE, Nader K (2002). Cellular and systems reconsolidation in the hippocampus. Neuron 36: 527–538.
Devauges V, Sara SJ (1991). Memory retrieval enhancement by locus coeruleus stimulation: evidence for mediation by beta-receptors. Behav Brain Res 43: 93–97.
Foltin RW, Haney M (2000). Conditioned effects of environmental stimuli paired with smoked cocaine in humans. Psychopharmacology (Berl) 149: 24–33.
Fuchs RA, Evans KA, Ledford CC, Parker MP, Case JM, Mehta RH et al (2005). The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine seeking in rats. Neuropsychopharmacology 30: 29.
Hearing MC, Schochet TL, See RE, McGinty JF (2010). Context-driven cocaine-seeking in abstinent rats increases activity-regulated gene expression in the basolateral amygdala and dorsal hippocampus differentially following short and long periods of abstinence. Neuroscience 170: 570–579.
Hermann D, Smolka MN, Wrase J, Klein S, Nikitopoulos J, Georgi A et al (2006). Blockade of cue-induced brain activation of abstinent alcoholics by a single administration of amisulpride as measured with fMRI. Alcohol Clin Exp Res 30: 1349–1354.
Hopkins WF, Johnston D (1984). Frequency-dependent noradrenergic modulation of long-term potentiation in the hippocampus. Science 226: 350–352.
Jasmin L, Narasaiah M, Tien D (2006). Noradrenaline is necessary for the hedonic properties of addictive drugs. Vascul Pharmacol 45: 243–250.
Ji XH, Cao XH, Zhang CL, Feng ZJ, Zhang XH, Ma L et al (2008). Pre- and postsynaptic beta-adrenergic activation enhances excitatory synaptic transmission in layer V/VI pyramidal neurons of the medial prefrontal cortex of rats. Cereb Cortex 18: 1506–1520.
Kampman KM, Dackis C, Lynch KG, Pettinati H, Tirado C, Gariti P et al (2006). A double-blind, placebo-controlled trial of amantadine, propranolol, and their combination for the treatment of cocaine dependence in patients with severe cocaine withdrawal symptoms. Drug Alcohol Depend 85: 129–137.
Kampman KM, Volpicelli JR, Mulvaney F, Alterman AI, Cornish J, Gariti P et al (2001). Effectiveness of propranolol for cocaine dependence treatment may depend on cocaine withdrawal symptom severity. Drug Alcohol Depend 63: 69–78.
Kim J, Song B, Hong I, Lee J, Park S, Eom JY et al (2010). Reactivation of fear memory renders consolidated amygdala synapses labile. J Neurosci 30: 9631–9640.
Kim JJ, Fanselow MS (1992). Modality-specific retrograde amnesia of fear. Science 256: 675–677.
Kroes MC, Strange BA, Dolan RJ (2010). Beta-adrenergic blockade during memory retrieval in humans evokes a sustained reduction of declarative emotional memory enhancement. J Neurosci 30: 3959–3963.
Kroes MC, Tona KD, Muller N, Den Ouden HE, Van Wingen GA, Fernandez G (2012) Beta-Adrenergic Blockade Affects the Neural Network of Extinction Learning and Prevents the Return of Fear in Humans. Program No. 708.08. 2012 Neuroscience Meeting Planner. Society for Neuroscience: New Orleans, LA; (2012).
LaLumiere RT, Niehoff KE, Kalivas PW (2010). The infralimbic cortex regulates the consolidation of extinction after cocaine self-adminstration. Learn Mem 17: 168–175.
Lee JL, Everitt BJ, Thomas KL (2004). Independent cellular processes for hippocampal memory consolidation and reconsolidation. Science 304: 839–843.
Ma X, Zhang JJ, Yu LC (2012). Post-retrieval extinction training enhances or hinders the extinction of morphine-induced conditioned place preference in rats dependent on the retrieval-extinction interval. Psychopharmacology (Berl) 221: 19–26.
McGaugh JL (2000). Memory-a century of consolidation. Science 287: 248–251.
Merlo AB, Izquierdo I (1967). The effect of catecholamines on learning in rats. Med Pharmacol Exp Int J Exp Med 16: 343–349.
Meyers RA, Zavala AR, Neisewander JL (2003). Dorsal, but not ventral, hippocampal lesions disrupt cocaine place conditioning. Neuroreport 14: 2127–2131.
Meyers RA, Zavala AR, Speer CM, Neisewander JL (2006). Dorsal hippocampus inhibition disrupts acquisition and expression, but not consolidation, of cocaine conditioned place preference. Behav Neurosci 120: 401–412.
Miller CA, Marshall JF (2004). Altered prelimbic cortex output during cue-elicited drug seeking. J Neurosci 24: 6889–6897.
Mueller D, Porter JT, Quirk GJ (2008). Noradrenergic signaling in infralimbic cortex increases cell excitability and strengthens memory for fear extinction. J Neurosci 28: 369–375.
Mueller D, Stewart J (2000). Cocaine-induced conditioned place preference: reinstatement by priming injections of cocaine after extinction. Behav Brain Res 115: 39–47.
Murchison CF, Zhang XY, Zhang WP, Ouyang M, Lee A, Thomas SA (2004). A distinct role for norepinephrine in memory retrieval. Cell 117: 131–143.
Narayanan RT, Seidenbecher T, Sangha S, Stork O, Pape HC. (2007). Theta resynchronization during reconsolidation of remote contextual fear memory. Neuroreport 18: 1107–1111.
Neisewander JL, Baker DA, Fuchs RA, Tran-Nguyen LT, Palmer A, Marshall JF. (2000). Fos protein expression and cocaine-seeking behavior in rats after exposure to a cocaine self-administration environment. J Neurosci 20: 798–805.
Olson VG, Heusner CL, Bland RJ, During MJ, Weinshenker D, Palmiter RD (2006). Role of noradrenergic signaling by the nucleus tractus solitarius in mediating opiate reward. Science 311: 1017–1020.
Otis JM, Dashew KB, Mueller D (2013). Neurobiological dissociation of retrieval and reconsolidation of cocaine-associated memory. J Neurosci 33: 1271–1281.
Otis JM, Mueller D (2011). Inhibition of β-adrenergic receptors induces a persistent deficit in retrieval of a cocaine-associated memory providing protection against reinstatement. Neuropsychopharmacology 36: 1912–1920.
Pavlov IP (1927) Conditioned Reflexes: An Investigation of the Physiological Activity of the Cerebral Cortex. Oxford University Press: Oxford, England.
Pedarzani P, Storm JF (1993). PKA mediates the effects of monoamine transmitters on the K+ current underlying the slow spike frequency adaptation in hippocampal neurons. Neuron 11: 1023–1035.
Ramirez DR, Bell GH, Lasseter HC, Xie X, Traina SA, Fuchs RA (2009). Dorsal hippocampal regulation of memory reconsolidation processes that facilitate drug context-induced cocaine-seeking behavior in rats. Eur J Neurosci 30: 901–912.
Rescorla RA (2004). Spontaneous recovery. Learn Mem 11: 501–509.
Sara SJ (2009). The locus coeruleus and noradrenergic modulation of cognition. Nat Rev Neurosci 10: 211–223.
Sara SJ, Segal M (1991). Plasticity of sensory responses of locus coeruleus neurons in the behaving rat: implications for cognition. Prog Brain Res 88: 571–585.
Wells AM, Lasseter HC, Xie X, Cowhey KE, Reittinger AM, Fuchs RA (2011). Interaction between the basolateral amygdala and dorsal hippocampus is critical for cocaine memory reconsolidation and subsequent drug context-induced cocaine-seeking behavior in rats. Learn Mem 18: 693–702.
Wouda JA, Diergaarde L, Riga D, van Mourik Y, Schoffelmeer AN, De Vries TJ (2010). Disruption of long-term alcohol-related memory reconsolidation: role of β-adrenoceptors and NMDA receptors. Front Behav Neurosci 4: 1–7.
Zhao LY, Shi J, Zhang XL, Epstein DH, Zhang XY, Liu Y et al (2010). Stress enhances retrieval of drug-related memories in abstinent heroin addicts. Neuropsychopharmacology 35: 720–726.
Zhou M, Kindt M, Joels M, Krugers HJ (2011). Blocking mineralocorticoid receptors prior to retrieval reduces contextual fear memory in mice. PLoS ONE 6: e26220.
Acknowledgements
This research was supported by DA027870 and a grant from the University of Wisconsin-Milwaukee Research Growth Initiative to DM. We thank Patrick Reilly for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
Rights and permissions
About this article
Cite this article
Otis, J., Fitzgerald, M. & Mueller, D. Inhibition of Hippocampal β-Adrenergic Receptors Impairs Retrieval But Not Reconsolidation of Cocaine-Associated Memory and Prevents Subsequent Reinstatement. Neuropsychopharmacol 39, 303–310 (2014). https://doi.org/10.1038/npp.2013.187
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/npp.2013.187
Keywords
This article is cited by
-
Repeated ketamine anesthesia during neurodevelopment upregulates hippocampal activity and enhances drug reward in male mice
Communications Biology (2022)
-
The role of hippocampus in memory reactivation: an implication for a therapeutic target against opioid use disorder
Current Addiction Reports (2022)
-
A ventral CA1 to nucleus accumbens core engram circuit mediates conditioned place preference for cocaine
Nature Neuroscience (2019)
-
Generation of silent synapses in dentate gyrus correlates with development of alcohol addiction
Neuropsychopharmacology (2018)
-
Differential Roles for L-Type Calcium Channel Subtypes in Alcohol Dependence
Neuropsychopharmacology (2017)